- Visibility 213 Views
- Downloads 27 Downloads
- Permissions
- DOI 10.18231/j.ijca.2021.090
-
CrossMark
- Citation
Anesthetic management of an achondroplastic dwarf with difficult airway and kyphoscoliosis for total abdominal hysterectomy
- Author Details:
-
Jyoti P Deshpande
-
Jyoti H Kale
-
Madhavi R Godbole
-
Tejaswini L Phalke *
Abstract
Achondroplasia is a common form of dwarfism and possesses multiple anesthetic challenges including securing of intravenous line, monitoring and calculating drug dosage, spine abnormality, difficulty in mask ventilation and endotracheal intubation, obesity, cardiopulmonary and neurological system abnormality. There is multiple systems involvement, therefore thorough preanesthetic check ups, investigations and planning for anesthesia is important. Here we came across 36 years old female patient, achondroplasic dwarf (height- 100cm) with thoracolumbar kyphoscoliosis, fused cervical spine, short neck and restricted neck movement with mild pulmonary restrictive disease for total abdominal hysterectomy. Patient also had complained of generalized weakness and fatigue. She had a limited neck extension and short neck possesses anticipated difficult intubation, therefore we planned awake fiberoptic intubation with smaller size endotracheal tube for airway management and general anesthesia in a patient with difficult airway and spine for total abdominal hysterectomy. As the spread of the drug in regional anesthesia is unpredicted, we planned general anesthesia with awake fiberoptic intubation to avoid the risk of neurological injury while extending the neck during laryngoscopy for tracheal intubation due to restricted neck movement.
Case History
36 years old, female patient, case of achondoplastic dwarfism had a uterine fibroid with heavy per vaginal bleeding posted for total abdominal hysterectomy. She stands 100cm and weighted 33 kg (BMI-33kg/m2). Patient had dyspnoea on exersion MMRC grade I since childhood. Achondroplasia was associated with thoracolumbar kyphoscoliosis, fused cervical spine, short neck and restricted neck movements with mild pulmonary restrictive disease. She did not have any significant past history of chronic illness or any surgeries.
On general examination, she was afebrile with pulse of 90/min, blood pressure of 108/74 mm Hg and respiratory rate 20/min. Breath holding time was 14 seconds. Mild pallor present. Her airway assessment revealed a large head and occiput, depressed nose, large tounge with receding mandible, short neck with marked limitation of neck extension, decreased submandibular distance ([Figure 1]). Mouth opening was adequate with mallampatti classification II. Examination of spine shows gross thoracolumbar kyphoscoliosis. Lower limb examination revealed a valgus deformity. Electrocardiography & Echocardiography was normal, pulmonary function test suggestive of mild restrictive disease. Chest radiograph shows scoliotic spine ([Figure 2]). Her blood reports showed mild anemia, other blood investigations were within normal limits. Ultrasonography demonstrated multiple intramural & submucosal fibroids and horseshoe shaped kidney. In view of her spinal abnormality, fused cervical spine, limited neck extension and expected difficult mask ventilation & intubation, awake fiberoptic intubation and general anesthesia with control ventilation was planned.


Written informed consent with high risk explained was obtained. NBM status was confirmed. 18G IV access was secured. Difficult intubation trolly including fiberoptic kept ready. Emergency resuscitation drugs, equipments & defibrillator were kept ready. In operating room, monitors (pulse oximetry, non invasive BP monitoring, ECG, temperature).
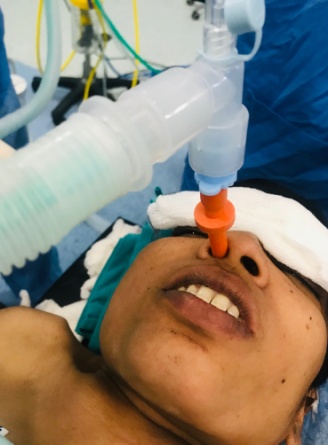
Inj.Glycopyrrolate 0.2mg intramuscularly was given 30 mins before induction in preoperative room, xylometazolin 0.1% nasal drops were given in both nostrils. Inj.Dexamethasone 0.2mg/kg IV and Inj.Hydrocort 2mg/kg IV given preoperatively. Difficult airway cart was prepared and toxic dose of local anesthetic was calculated. She was nebulised with topical lignocaine 4% 2ml followed by spraying of the base of tongue and posterior pharyngeal wall with 10% lignocaine before procedure. Inj. Dexmedetomidine 0.4mcg/kg IV given before procedure. Superior laryngeal nerve block were given with inj.Lignocaine 2% 1.5ml on both sides & transtracheal block given with inj.Lignocaine 2% 4ml. Before insetion of fiberoptic bronchoscope patency of the nostrils were checked. Patient was oxygenated with nasal airway with 6 mm connector from opposite nostril by bains circuit ([Figure 3]). Awake fiberoptic nasal intubation was done using spray as you go technique with maintaining cervical spine stability throughout and intubation was successful in first attempt. After securing the airway, intravenous induction was done with Inj.Propofol 2mg/kg IV and Inj.Vecuronium 0.1mg/kg IV and patient was taken on mechanical ventilator. Inj. Fentanyl 2mcg/kg IV & Inj. Paracetamol 15 mg/kg IV given as an analgesia. Surgery was lasted for 2 hours. Blood loss was minimal. Patient was hemodynamically stable throughout the procedure. She was reversed with Inj.Neostigmine 0.05mg/kg IV and Inj. Glycopyrrolate 8mcg/kg IV and extubated uneventfully. She was shifted to postoperative care unit. Postoperative analgesia was maintained with inj. Paracetamol 15 mg/kg IV TDS.
Discussion
Achondroplasia is the most common cause of dwarfism caused by gain of function mutation in fibroblast growth factor receptor 3 (FGFR3) gene. 80% mutations are the result of sporadic mutation while 20% are autosomal dominant. Incidence is approximately 0.5-1.5 in 10,000 newborns.[1] Females are more affected than the male.[2] Clinically, characteristic symptoms of disproportionate dwarfism is relative large head, midfacial hypoplasia. Achondroplasia affects multiple systems, primary or secondary to consequences of other organ systems affected.
A general recommendation regarding the ideal anaesthetic technique cannot be given, as both general and regional anaesthesia present potential problems.[3] Therefore, individual decisions are necessary.
Problems during general anesthesia includes Excessive anxiety,[4], [5] difficult intravenous access, difficult Laryngoscopy and endotracheal intubation[6], [7] and difficult mask ventilation,[8], [9] risk for cervico-medullary compression or rather spinal cord ischemia (reported sudden death events), ten-fold increased cardiovascular risk – with a maximum between 25 and 35 years of age,[10], [11] eight-fold higher obesity rate potentiating the effects of the existing problems and increased incidence of sleep apnea (obstructive and/or central)[9], [10] – rarely secondary pulmonary hypertension, restrictive lung diseases already at an early age,[8], [9] chronic respiratory infections[1] tendency toward hypersalivation. Increase incidence of gastro-oesophageal reflux disease in dwarf patients therefore there is a risk of aspiration.
Despite of all these problems during general anesthesia, we preferred general anesthesia over regional, due to the anatomical changes in the spine and the craniocervical junction as well as an increased incidence of hydrocephalus, spinal anesthesia is relatively contraindicated.
Neuraxial regional anesthesia is considered to be technically difficult due to narrow spinal canal/stenosis, reduced epidural space, kyphoscoliosis, vertebral body deformities.[12] There are chances of potential neurological failures after regional anesthesia. In some cases, epidural anaesthesia was carried out successful. There are reports about accidental dural punctures, increased risk of venous puncture,[13] difficulties in advancing the catheter, irregular or unpredictable spread of anesthesia.[13] Epidural anesthesia should be preferred because of the possibility of titration. The conus medullaris is often positioned lower than usual.[14] In most cases, injection of local anaesthetic into the caudal canal is easier in the case of paediatric patients.
Also spinal anesthesia even when applied successfully,[15] carries the risk of failure of block as a result of unpredicted spread of the subarachnoid block due to anatomical deformity of spine.
While performing awake fiberoptic intubation, it is important to allay the anxiety of patient as these patients are highly anxious, we gave dexmedetomidine intravenously before procedure with vitals monitoring. Dexmedetomidine is a highly selective alpha 2 adrenoreceptor agonist and has a several unique properties, including sedation, anxiolysis, analgesia, hemodynamic stability and easy arousability. Dexmedetomidine demonstrates minimal respiratory depression[16] even at a higher doses and also decrease salivary secretions, which are desirable during awake FOI.
Greatest care is required to prevent damage caused by positioning in case of anatomical abnormalities like khyphoscoliosis, lower limb valgus deformity, joint contractures and some patients are not able to lie supine.[17] There are incidence of brachial plexus injuries during positioning in these patients. Case reports on damages caused by positioning do exist (e.g. two cases of brachial plexus palsy).[18] Therefore we kept pillows underneath bilateral lower limb as a patient had joint contractures.
Compared to the size of the body, the head is relatively large & because of increase in body surface area they are more prone for hypothermia. Therefore, temperature monitoring and prevention of hypothermia is important in these patients.
Therefore in our case, we planned awake fiberoptic intubation as she had predicted difficult airway and to avoid neurological injury due to cervical spine extension during laryngoscopy, with all preoperative preparation for difficult mask ventilation and difficult intubation.
Conclusion
Thorough knowledge, detailed planning and skilled anesthesiologist to perform awake fiberoptic bronchoscopy are necessary for successful outcome of difficult airway management in achondroplastic patient.
Source of Funding
None.
Conflict of Interest
The author declares no conflict of interest.
References
- Berkowitz I, Raja S, Bender K, Kopitz S. Dwarfs. Pathophysiology and anesthetic implications. Anesthesiology. 1990;73:739-59. [Google Scholar]
- Huang J, Babins N. Anesthesia for cesarean delivery in a achondroplastic dwarf: a case report. AANA J. 2008;76(6):435-6. [Google Scholar]
- Sukanya M, Nilanjan D, Gomber K. Emergency cesarean section in a patient with achondroplasia: an anesthetic dilemma. J Anesth Clin Pharmacol. 2007;23(3):315-8. [Google Scholar]
- Krishnan B, Eipe N, Korula G. Anaesthetic management of a patient with achondroplasia. Paediatr Anaesth. 2003;13(6):547-9. [Google Scholar]
- Simmons K, Hashmi S, Scheuerle A, Canfield M, Hecht J. Mortality in babies with achondroplasia: revisited. Birth Defects Res A Clin Mol Teratol. 2014;100(4):247-9. [Google Scholar]
- Abrao M, Silveira VD, Barcellos C, Cosenza R, Cameiro J. Anesthesia for bariatric surgery in an achondroplastic dwarf with morbid obesity. Rev Bras Anesthesiol. 2009;59(1):82-6. [Google Scholar]
- Trikha A, Goyal K, Sadera GS, Singh M. Combined spinal epidural anaesthesia for vesico-vaginal fistula repair in an achondroplastic dwarf. Anaesth Intensive Care. 2002;30(1):96-98. [Google Scholar]
- Ottononello G, Villa G, Moscatelli A, Diana M, Pavanello M. Noninvasive ventilation in a child affected by achondroplasia respiratory difficulty syndrome. Paediatr Anaesth. 2007;17(1):75-9. [Google Scholar]
- Sisk E, Heatley D, Borowski B, Leverson G, Pauli R. Obstructive sleep apnea in children with achondroplasia: surgical and anesthetic considerations. Otolaryngol Head Neck Surg. 1999;120(2):248-54. [Google Scholar]
- Srinivas S, Ramalingam R, Manjunath C. A rare case of percutaneous coronary intervention in achondroplasia. J Invasive Cardiol. 2013;25(6):136-8. [Google Scholar]
- Wynn J, King T, Gambello M, Waller D, Hecht J. Mortality in achondroplasia study: a 42-year follow-up. Am J Med Genet A. 2007;143(21):2502-11. [Google Scholar]
- Cohen S. Anesthesia for cesarean section in achondroplastic dwarfs. Anaesthesiology. 1980;52(3):264-6. [Google Scholar]
- Morrow M, Black I. Epidural anaesthesia for caesarean section in an achondroplastic dwarf. Br J Anaesth. 1998;81(4):619-21. [Google Scholar]
- Dubiel L, Scott G, Agaram R, Mcgrady E, Duncan A, Litchfield K. Achondroplasia: anaesthetic challenges for caesarean section. Int J Obstet Anesth. 2014;23(3):274-8. [Google Scholar]
- Derenzo J, Vallejo M, Ramanathan S. Failed regional anesthesia with reduced spinal bupivacain dosage in a parturient with achondroplasia presenting for urgent cesarean section. Int J Obstet Anesth. 2005;14(2):175-8. [Google Scholar]
- Ebert T, Maze M. Dexmedetomidine: Another arrow for the clinician's quiver. Anesthesiology. 2004;101:568-70. [Google Scholar]
- Hecke D, DeVille A, Linden P, Faraoni D. Anaesthesia and orphan disease: a 26-year-old patient with achondroplasia. Eur J Anaesthesiol. 2013;30(12):776-9. [Google Scholar]
- Mayhew J, Katz J, Miner M, Leiman B, Hall I. Anaesthesia for the achondroplastic dwarf. Can Anaesth Soc J. 1986;33(2):216-21. [Google Scholar]
How to Cite This Article
Vancouver
Deshpande JP, Kale JH, Godbole MR, Phalke TL. Anesthetic management of an achondroplastic dwarf with difficult airway and kyphoscoliosis for total abdominal hysterectomy [Internet]. Indian J Clin Anaesth. 2021 [cited 2025 Oct 09];8(3):475-478. Available from: https://doi.org/10.18231/j.ijca.2021.090
APA
Deshpande, J. P., Kale, J. H., Godbole, M. R., Phalke, T. L. (2021). Anesthetic management of an achondroplastic dwarf with difficult airway and kyphoscoliosis for total abdominal hysterectomy. Indian J Clin Anaesth, 8(3), 475-478. https://doi.org/10.18231/j.ijca.2021.090
MLA
Deshpande, Jyoti P, Kale, Jyoti H, Godbole, Madhavi R, Phalke, Tejaswini L. "Anesthetic management of an achondroplastic dwarf with difficult airway and kyphoscoliosis for total abdominal hysterectomy." Indian J Clin Anaesth, vol. 8, no. 3, 2021, pp. 475-478. https://doi.org/10.18231/j.ijca.2021.090
Chicago
Deshpande, J. P., Kale, J. H., Godbole, M. R., Phalke, T. L.. "Anesthetic management of an achondroplastic dwarf with difficult airway and kyphoscoliosis for total abdominal hysterectomy." Indian J Clin Anaesth 8, no. 3 (2021): 475-478. https://doi.org/10.18231/j.ijca.2021.090
