- Visibility 146 Views
- Downloads 27 Downloads
- Permissions
- DOI 10.18231/j.ijca.2020.083
-
CrossMark
- Citation
Comparison of IV magnesium sulphate and IV esmolol in attenuating hemodynamic extubation response after general anesthesia
- Author Details:
-
Chetan Gopal Agrawal
-
Suchita Joshi Khadke *
Abstract
Background: Tracheal extubation is associated with a 10–30% increase in arterial pressure and heart rate that could be detrimental in patients with hypertension, ischemic heart disease, and cerebrovascular disease. A reliable technique for rapid & smooth extubation with stable hemodynamics is still not fully evolved.
Aims: To compare the effect of intravenous magnesium sulphate and esmolol for attenuating hemodynamic response to extubation after general anesthesia.
Setting and Design: Prospective, randomized, double blind study conducted at tertiary care hospital.
Methods: Sixty adult subjects undergoing major surgery were randomly divided into 2 groups. Group M received magnesium sulphate 40 mg/kg & Group E received esmolol 0.6 mg/kg IV infusion over 5 minutes before extubation. Heart rate, systolic & diastolic blood pressure, mean arterial pressure, rate pressure product, pain score & sedation score were compared from extubation till 15 minutes after extubation.
Statistical Analysis: Students ‘t’ test used for continuous variables & Chi Square test for categorical data.
Results: 21.82% fall in heart rate was observed in group M with a plateau at 10 -15 minutes compared to 37.07% in group E with a peak at 15 minutes (p=0.0150). Fall in Systolic blood pressure was 18.86% in group M & 21.15% in group E (p=0.4298). Rate pressure product was significantly lower in group E (50.40%) than group M (36.66%). Postoperative pain score was significantly less in group M compared to group E (p<0.0001).
Conclusion: Magnesium sulphate provides better hemodynamic stability with postoperative analgesia compared to esmolol when used for attenuating hemodynamic response to extubation.
Introduction
Tracheal extubation is an important event in course of general anesthesia. It is usually performed under lighter plane of anesthesia.[1], [2] Extubation irritates airways, causing cough or strain, both of which are known to increase systolic, diastolic and arterial pulse pressure. Coughing can lead to increases in intrathoracic pressure which can interfere with venous return to the heart. Reflex sympathetic discharge caused by epipharyngeal and laryngopharyngeal stimulation producing significant increase in heart rate and arterial pressure that may persist into recovery period.[3], [4], [5], [6] Lowrie and colleagues demonstrated a significant increase in the plasma concentration of adrenaline during tracheal extubation after major elective surgery.[1], [3], [4], [6] If specific measures are not taken to prevent hemodynamic response, the heart rate can increase from 26% to 66% and systolic blood pressure from 36% to 45%in susceptible patients.[3], [7] Dyson and colleagues demonstrated increases in arterial pressure and heart rate lasting 5–15 min in 70% of the patients.[2], [3], [8], [9]
Hypertensive subjects may exhibit an exaggerated response to awakening and tracheal extubation compared with that seen in normotensive patients.[2], [7] In patients with coronary artery disease, the hemodynamic response to extubation may upset the balance between myocardial oxygen supply and demand, resulting in myocardial ischemia, left ventricular dysfunction, cardiac dysrhythmias, cerebral hemorrhage. They may experience a 40–50% reduction in ejection fraction.[5], [9] After intracranial surgery, 91% of patients became hypertensive when the volatile anesthetic was discontinued ending after the trachea was extubated. This sudden increase may result in either herniation of brain contents or decrease in cerebral perfusion pressure, leading to cerebral ischemia.[2], [4] Increased risk of cerebral hemorrhage and pulmonary edema is observed in parturient with gestational hypertension undergoing caesarean section under general anesthesia secondary to significant increases in mean arterial and pulmonary artery pressures of about 45 and 20 mm Hg respectively.[1], [2], [3], [9]
A variety of drugs have been recommended for the control of hemodynamic events during extubation. Esmolol, is a short acting, highly cardio-selective beta-adrenergic receptor antagonist rapidly metabolized by plasma esterase.[1], [10] It provides hemodynamic stability during laryngoscopy, tracheal intubation and extubation more effectively compared to fentanyl, alfentanil, nitroglycerine, diltiazem and lidocaine.[1], [11] The rapid onset and short duration of action (T1/2 = 9 min) of esmolol make it an ideal agent to prevent acute increases in heart rate and arterial pressure which occur at extubation.[1], [2], [8] The role of magnesium in blunting the hemodynamic response to endotracheal intubation and extubation is evolving.[1], [3], [10] We undertook this randomized, prospective, double blind study to compare IV esmolol and IV magnesium sulphate (MgSo4) for attenuating hemodynamic response to extubation after general anaesthesia (GA).
Materials and Methods
This prospective, randomized, double blind, comparative study was carried out at tertiary care hospital after approval from institutional ethics committee. The study was conducted according to guidelines laid down by Helsinki Declaration of 1975, as revised in 2000; it is registered to CTRI (No. CTRI/2018/05/014202). Sixty adult patients of American Society of Aaesthesiologist (ASA) Grade 1 and 2, age 20 to 55 years, weight 40-70 kg of both sexes undergoing elective surgery under general anesthesia with endotracheal intubation were enrolled in the study. Informed, written consent was obtained in vernacular language. Screening procedure includes history, general & systemic examination and airway assessment. Baseline & special investigations were carried out as per coexisting disease condition.
Patients refusing to participate in the study, allergy to study drugs, uncontrolled cardiovascular disease, hepatic or renal disease, bronchospastic disease, ASA grade 3 or more, emergency surgery, patients taking any adrenergic or psychotropic drugs, difficult intubation, myasthenia gravis or musculoskeletal disorder were excluded from the study. All patients were kept nil by mouth 6-8 hours before surgery. In the operating room baseline pre-operative heart rate (HR), SBP (Systolic Blood Pressure), DBP (Diastolic Blood Pressure), MAP (Mean Arterial Pressure), RPP (Rate Pressure Product), RR (Respiratory Rate), ECG, SPO2 were recorded. All the patients were pre-medicated with IV midazolam- 0.02mg/kg, pentazocine- 0.5mg/kg, ondansetron- 4 mg IV. After preoxygenation, induction of general anesthesia was done using IV thiopentone sodium (2.5%) 4-6 mg/kg till the loss of eyelid reflex & suxamethonium- 2mg/kg IV, trachea was intubated with appropriate size endotracheal tube, tube secured. Anesthesia was maintained with oxygen, nitrous oxide, isoflurane and intermittent IV atracurium. Fluids & blood loss was replaced with IV fluid or blood as appropriate. IV paracetamol infusion was given for postoperative analgesia.
At the end of surgery patients were randomly allocated into two groups i.e. 30 subjects in each group using computer generated randomization list with allocation ratio 1:1. Group M received IV magnesium sulphate – 40mg/kg started 5 minutes before extubation and Group E received IV esmolol – 0.6 mg/kg started 5minutes before extubation. Both drugs were diluted in100 ml normal saline & administered over 5 minutes. The infusion was prepared by anaesthesiologist not involved in data collection. Thus study participants & observer were blind to the study drug. Unblinding was done after complete data collection before statistical analysis. The infusion was stopped if patient develops any significant side effect & treated accordingly. Neuromuscular blockade reversed with IV neostigmine (0.05 mg/kg) and glycopyrrolate (8µg/kg). Patients were extubated after confirming adequate reversal of neuromuscular block. Study vitals (SBP, DBP, MAP, RPP, RR, ECG, SPO2, sedation score, VAS) were recorded before extubation, at the time of extubation, 1, 3, 5, 10 & 15 minutes after extubation. Sedation was measured by Ramsay sedation score (Score Responsiveness 1 - patient is anxious and agitated or restless, or both 2-cooperative, oriented and tranquil, 3- Patient responds to commands only, 4 -brisk response to light glabellar tap or loud auditory stimulus, 5 -a sluggish response to light glabellar tap or loud auditory stimulus, 6-no response). Postoperative pain was measured using 10 cm Visual Analogue Pain Scale (VAS 0-no pain, 10- Intolerable pain).[12], [13]
The patients were monitored at post anesthesia care unit for complications like arrhythmias, bradycardia, respiratory depression, muscle weakness, nausea & vomiting before shifting to ward.
Sample size of 30 subjects in each group was calculated from mean & standard deviation of reference study with power 80%, difference in mean arterial pressure >20% of baseline value considered to be significant using Open Epi software Version 3.01 Updated 2013/04/06.(https://www.openepi.com)(Value of p<0.05 – statistically significant). Data was tabulated in MS excel and analysed using one tailed Students t test (paired or unpaired as appropriate) for continuous variables & Chi Square test for categorical data with statistical software SPSS Version 20. (Statistical Package for the social Sciences)(www.ibm.com). Interim analysis was done after completion of 50% of sample size.
Results
This prospective, randomized, double blind study was designed to compare IV Magnesium Sulphate (Group M) and IV Esmolol (Group E) in attenuating hemodynamic extubation response after general anesthesia. Sixty-five cases were screened & enrolled in the study. Three patients in Group E and 2 patients in Group M were excluded from statistical analysis for gross protocol deviations. Thus, total sixty patients were included in the study.
Both the groups were comparable with respect to age, weight, gender, ASA physical status & type of surgery ([Table 1]). The surgical procedures include appendectomy, cholecystectomy, lipoma excision, exploratory laparotomy, hernia repair, burn escharotomy, pleomorphic adenoma excision, tonsillectomy, endoscopic sinus surgery.
Baseline & before extubation HR, SBP, DBP, MAP, RPP, VAS & sedation score were comparable between group E & group M ([Table 2]). At the time of extubation significantly reduction in vital parameters was observed in both the groups compared with the baseline values. The fall in HR & RPP was more in esmolol group as compared to Magnesium group (P value 0.018 for HR and 0.013 for RPP). At one minute after extubation reduction in HR and RPP was statistically extremely significant between group E & group M (p =0.0017 for HR and 0.0014 for RPP) ([Table 2]). Five minutes after extubation HR, SBP and RPP were significantly reduced in both groups compared to baseline values. The difference between group E & group M was statistically extremely significant (P value 0.0457 for SBP, P<0.0001 for HR and RPP) ([Table 2]). Similarly, at ten & fifteen minute after extubation HR, SBP and RPP were reduced in both groups compared to baseline values. DBP & MAP were significantly reduced in both groups compared with the baseline values but the difference between the two groups was not statistically significant ([Table 2]).
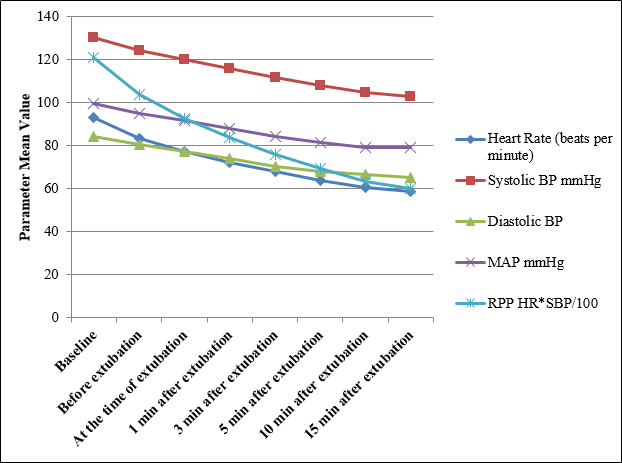
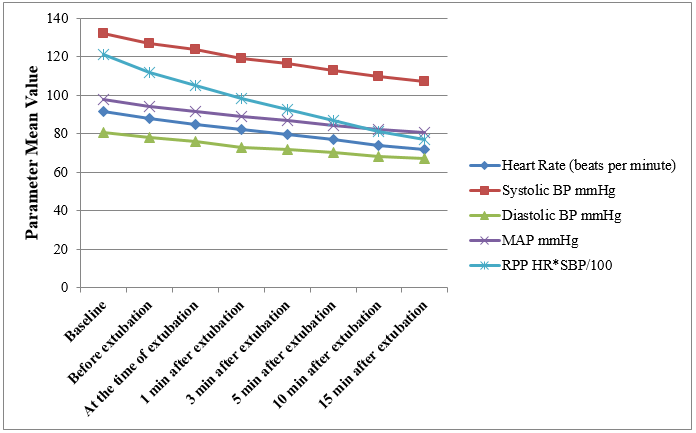
[Figure 1], [Figure 2] shows mean change in vital parameters in Group E & Group M respectively before extubation, at the time of extubation, 1, 3, 5, 10 & 15 minutes after extubation from baseline. In group E average fall in HR was 37.07%, SBP-21.15%, DBP-22.39%, MAP-20.29% and RPP-50.40%. The effect persisted till 15 minutes after extubation whereas in group M the average fall in HR was 21.82%, SBP-18.86%, DBP-16.75%, MAP-17.76% & RPP-36.66%. Thus the deviation in vital parameters from baseline was less in Group M compared to Group E, the difference in HR & RPP was statistically significant (p value 0.0150 for HR & 0.0435 for RPP).
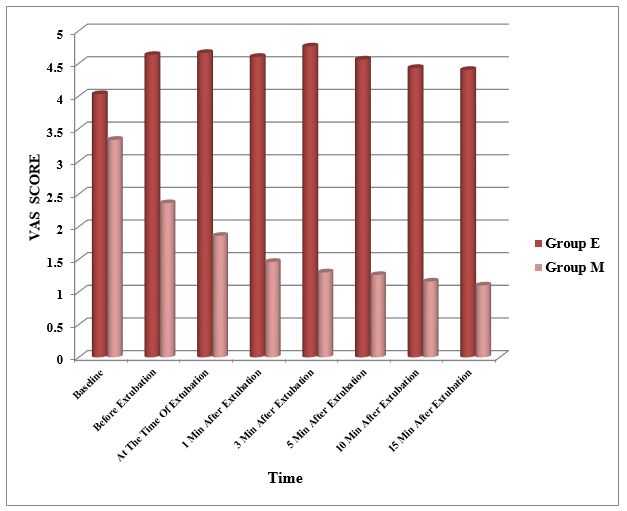
[Figure 3] depicts the change in VAS score in study groups. VAS score was slightly increased in group E as compared to baseline 4.03 (1.88) with a peak of 4.76 (1.38) at 3 minutes after extubation. VAS was persistently above 4 for the remaining observation period in group E; whereas in group M, VAS score was significantly reduced from baseline 3.33 (1.60) to 1.30 (0.46) at 3 minutes after extubation. The VAS score was persistently below 2 for the remaining observation period in group M (P<0.0001) ([Figure 3]). Thus, increase in VAS score was by 3.47% in group E whereas it was decreased by 24.65% in group M which is statistically extremely significant (P<0.0001).
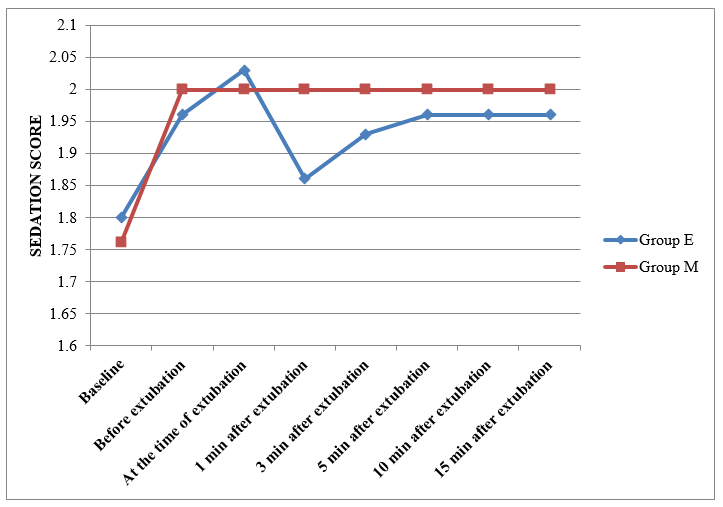
[Figure 4] shows change in sedation score in study groups. The sedation score was comparable between both groups except at 1 minute after extubation where it was increased in group M by 13.63% as compared to 8.88% (p<0.05) in group E. The sedation score remained at < 2 for rest of the observation period in both the groups & was comparable to baseline in each group.
No major adverse effects like allergic reactions, cardiac arrhythmias, drowsiness, dizziness, delayed recovery or skeletal muscle weakness was observed in any of the study group.
| Demographic Parameters | Group E N (%) | Group M N (%) | P value |
| Age (Years) | 36.86(13.26) | 41.03 (15.91) | *0.27 |
| Weight (Kg) | 60.50 (8.83) | 60.67 (7.48) | *0.93 |
| Gender (Male/Female) | 9/21 | 11/19 | †0.784 |
| ASA grade I/II | 25/5 | 23/7 | †0.748 |
| Parameter | Study group | Baseline | Before extubation | At the time of extubation | One minute after extubation | Five minute after extubation | Ten minute after extubation | Fifteen minute after extubation |
| Heart Rate (beats/ Minute) | Group E | 92.86 (14.11) | 83.26 (11.4) | 77.13 (10.55) | 72.10 (9.85) | 63.96 (8.67) | 60.67 (7.16) | 58.43 (6.05) |
| Group M | 91.50 (16.00) | 87.83 (14.98) | 84.96 (14.27) | 82.16 (13.58) | 76.76 (11.87) | 73.9 (10.85) | 71.53 (10.22) | |
| P value | 0.727- NS | 0.189-NS | 0.018- S | 0.0017-S | < 0.0001-S | <0.0001-S | <0.0001-S | |
| Systolic BP mmHg | Group E | 130.13(11.94) | 124.23(11.49) | 119.80 (10.26) | 115.90 (9.26) | 107.90(7.94) | 104.40(7.41) | 102.60 (6.19) |
| Group M | 132.13(13.69) | 126.76(12.59) | 123.06 (11.99) | 119.26(11.12) | 112.60 (9.78) | 109.6 (9.63) | 107.20 (9.09) | |
| P value | 0.549- NS | 0.419-NS | 0.261 –NS | 0.207- NS | 0.0457-S | 0.022-S | 0.0257-S | |
| Diastolic BP mmHg | Group E | 83.96(10.68) | 80.50(9.72) | 77.13(8.59) | 73.90 (7.77) | 67.83 (6.808) | 66.40 (6.67) | 65.16 (6.48) |
| Group M | 80.80 (10.79) | 78.03 (10.24) | 75.83 (9.31) | 73.00 (8.54) | 70.233 (7.66) | 67.96 (7.79) | 67.26 (9.01) | |
| P value | 0.25- NS | 0.342-NS | 0.576-NS | 0.671- NS | 0.204-NS | 0.406 -NS | 0.304-NS | |
| Mean Arterial Pressure mmHg | Group E | 99.36 (11.04) | 95.06 (9.63) | 91.40 (8.467) | 87.93 (7.50) | 81.23 (6.26) | 79.10 6.059) | 79.10 (6.05) |
| Group M | 97.93(11.14) | 94.16 (10.45) | 91.60 (9.44) | 88.83 (8.59) | 84.33 (7.401) | 81.93 (7.38) | 80.53 (7.31) | |
| P value | 0.61- NS | 0.73-NS | 0.931-NS | 0.667- NS | 0.0852-NS | 0.109 -NS | 0.411-NS | |
| Rate Pressure Product (HR*DBP/100) | Group E | 151.04 (16.08) | 103.58(17.44) | 92.54 (15.22) | 83.67 (13.35) | 69.12 (10.90) | 63.37 (8.84) | 60.03 (7.78) |
| Group M | 121.27 (26.70) | 111.59 23.31) | 104.78 (21.46) | 98.20 (19.57) | 86.61(16.25) | 81.14 (14.86) | 76.81 (13.50) | |
| P value | 0.32- NS | 0.13 -NS | 0.013-S | 0.0014-S | < 0.0001-S | < 0.0001-S | <0.0001-S |
Discussion
Almost all tracheal intubations are performed with the expectation of subsequent extubation. The increases in heart rate and arterial pressure associated with tracheal extubation mimic in size with those associated with laryngoscopy and tracheal intubation.[2], [9] But endotracheal intubation is performed in fully anesthetized state. Whereas tracheal stimulation & hemodynamic changes of extubation occurs after emergence from anesthesia with associated pain of the wound & other factors. A closed claims analysis of the American Society of Anesthesiologists database revealed that the claims for death or brain damage associated with induction of anesthesia decreased from 62% of perioperative claims in 1985–1992 to 35% in 1993-1999 while death or brain damage during maintenance, extubation, and recovery remained almost the same.[9] Thus, the potential for deleterious hemodynamic events to follow extubation, should not be ignored & it seems reasonable to attempt to minimize the hemodynamic response to tracheal extubation in all patients.
In this study we compared magnesium sulphate and esmolol with respect to their effects on hemodynamic responses during tracheal extubation in patients undergoing major surgery under general anesthesia. Our findings suggest that mean HR, SBP & RPP recorded a significant decrease in both magnesium sulphate and esmolol group immediately after tracheal extubation. The groups showed insignificant fall in MAP and DBP. This trend continued until 15 minutes post-extubation. RPP is a major determinant of myocardial oxygen consumption (MVO2). Levels of RPP in excess of 20000 are more commonly associated with angina pectoris &/or myocardial ischemia.[11], [14] When HR & rate pressure product was compared between esmolol and MgSo4 group, there seemed to have significant differences at respective time intervals. VAS was significantly lower with magnesium sulphate after extubation. We did not find differences between the groups in terms of Ramsay Sedation score. However, patients of magnesium sulphate group had a better profile than esmolol group. The advantage of magnesium is attributed to the fact that it does not result in bradycardia & provide postoperative analgesia.
Esmolol attenuates the tachycardic response to extubation more effectively than hypertensive response. Multiple studies have shown similar results.[11], [15] The use of esmolol before extubation may be beneficial in patients with ischemic heart disease and good left ventricular function, especially in the presence of borderline hypertension. The combination of neostigmine-glycopyrrolate mixture and esmolol with their possible synergistic bradycardic and hypotensive action may be disadvantageous to patients in whom cardiac depression is undesirable. The average fall in HR was 37.07% and fall in RPP was 50.40% from baseline till 15 minutes after extubation with esmolol. Thus the deviation in vital parameters from baseline was more with esmolol. We would advise caution when using esmolol during extubation unless the patient has received atropine or glycopyrrolate (as part of the reversal of neuromuscular blockade) because tracheal stimulation may produce profound bradycardia. We preferred lower dose of esmolol that is sufficient to produce attenuation without much bradycardia & hypotension. Moderate reduction in HR with MgSo4 is safe & beneficial in this situation. Although esmolol is selective adrenergic blocker & less likely to provoke bronchospasm, it does not have bronchodilator effect like that of MgSo4.[9]
The indications of magnesium sulphate in anesthesia are increasing over the years to include situations out of the gynecological field. The stress of intubation and extubation is associated with catecholamine release. Magnesium inhibits this catecholamine release from the adrenergic nerve endings and from the adrenal medulla. Along with sympatholysis it also blocks noxious stimuli, causes, coronary vasodilatation & improves contractility. It reduces incidence of arrhythmias & has been used successfully in serious atrial & ventricular arrhythmias, Torsade’s de pointes arrhythmia or status asthmaticus.[16] It causes a reduction in HR, BP, blood loss and duration of major head & neck surgery.[17] It is also effective in hypotensive anaesthesia and in reducing the requirement of anaesthetics, analgesics and muscle relaxants.[18] When magnesium is administered before induction, it prevents succinylcholine-induced increase in potassium levels & limits muscular fasciculation.[9], [19] The reduction in MAC of volatile anesthetics can be as high as 60%.[16], [20] It is a complementary drug in the treatment of hypertensive episodes during the surgical treatment of pheochromocytoma.[19] Magnesium sulphate can be recommended as an adjuvant during general anaesthesia for caesarean section to avoid perioperative awareness and pressor response.[21] The increase in HR with magnesium sulphate in previous studies could be attributed to the inhibition of action of acetylcholine on the heart.[17], [19], [22] A 40 mg/kg dose has shown similar efficacy to that of 10 µg/kg of alfentanil as well as greater effectivity than 1.5 mg/kg of lidocaine.[19] In many of its actions magnesium is likened to a physiological calcium antagonist.[23] It blocks calcium entry in the vascular smooth muscles via voltage & receptor operated receptor channels & diminishes the reactivity of these cells to pressor agents. Activation of membrane Ca-ATPase and Na-KATPase is involved in transmembrane ion exchanges during depolarization and repolarization phases, thus acting as a cell membrane stabilizer and also as an intracytoplasmic organelles stabilizer. This calcium inhibitory effect of Mg causes central arteriolar vasodilatation and acts against vasospasm.[23] Thus from a cardiovascular perspective, magnesium appears to be a very safe drug; a therapeutic ratio of at least 10:1 with plasma concentrations of 6 mmol/litre being described as haemodynamically safe.[24]
The effect of magnesium on perioperative analgesic requirement was first evaluated by Koinig and colleagues.[6] This is also confirmed in a study done by Shulz-Stubher et al.[25] Recent study suggested that NMDA receptor antagonism inhibits induction & maintenance of central sensitization after nociceptive stimuli&has the potential to prevent pain.[24] Magnesium antagonizes NMDA receptors in the CNS. This effect of MgSo4 on NMDA receptors is beneficial for alleviating postoperative pain & shivering in addition to lower incidence of PONV.[3], [4] Magnesium has been used successfully to potentiate opioid analgesia and in treating neuropathic pain in experimental studies. In a double blind study patients receiving infusion of magnesium sulphate had 30-40% morphine-sparing effect, lower pain score, less discomfort, less subjective sleep disturbances than controls.[16], [24], [25], [26] Decreased opioid consumption after surgery can be associated with less postoperative complications such as nausea and vomiting.[16] It enhances the activity of local anaesthetic agents. Another mechanism could involve the reduction of catecholamine released through sympathetic stimulation by which magnesium might decrease peripheral nociceptor sensitization & stress response to surgery. Various routes of magnesium administration have been shown to effectively control postoperative sore throat.[16] Magnesium sulphate administration may be useful for preventing thromboembolic complications after surgery.[16] Inhibitory action on smooth muscle contraction, histamine release from mast cells & inhibition of acetylcholine release from cholinergic nerve terminals along with modulation of calcium ion transport at cellular level has also been shown to relax bronchial smooth muscles & may be beneficial in preventing respiratory complications in postoperative period. It is a good bronchodilator.[27], [28] Thus, MgSo4 can contribute to improvement in the outcome of surgical patients. [28]
Although magnesium may potentiate neuromuscular blockade, it was assumed that the administration of single bolus dose magnesium sulphate did not prolong muscle relaxation.We did not observe any prolongation of neuromuscular blockade by magnesium sulphate; all the cases in our study were extubated uneventfully immediately after surgery. No patient was delayed to resume ventilation at the end of surgery as confirmed by a peripheral nerve stimulator at the wrist in previous studies.[28], [29] When magnesium is injected after full recovery from NMB, it is likely that the resulting recurarization can be reversed by neostigmine.[30] The minimum plasma magnesium concentration reported to block neuromuscular transmission is around 5mmol/l which is much higher than the clinically relevant concentrations of this drug. The normal physiological plasma magnesium concentration is around 0.8mmol/l. A dose of intravenous magnesium 50mg/kg is equivalent to 0.2mmol/kg and would be expected to increase the plasma magnesium concentrationbyabout1mmol/l. In addition, previous studies in which similar magnesium regimen was used, has noted almost 1.4–1.8 times increase in serum magnesium concentrations.[21] It was also reported that intra- and extracellular magnesium concentration do not accurately predict magnesium levels in other body tissues. However, in the presence of a non-depolarizing muscle relaxant, neuromuscular transmission becomes much more sensitive to the effect of magnesium and the slope of the dose–response curve becomes steeper; hence it is used with caution in muscle disease like myasthenia gravis and muscular dystrophies.[28]
We studied patients in ASA physical status I & II. This population was chosen to ensure the safety of the initial evaluation of the effects of MgSo4 in this setting. Our finding cannot be extrapolated in patients with hypertension, IHD or difficult airway. We have not used invasive methods of recording blood pressure or pulmonary artery pressure, so we could not measure beat to beat fluctuation of BP. One of the limitations of our study is that we did not measured serum magnesium concentrations in our study. Further studies are required to evaluate the advantage, other beneficial effects, and safety of magnesium sulphate in comparison with other drugs when used for the purpose of attenuating the hemodynamic changes associated with extubation in patients with CAD and cerebrovascular disease.
Conclusion
Both esmolol and magnesium had favorable outcomes in preventing cardiovascular responses to tracheal extubation. However, reduction in heart rate & rate pressure product was below the baseline values with esmolol. MgSo4 along with its analgesic and sedative property is found to be more beneficial & safer than esmolol after general anesthesia. Thus our results show that magnesium sulphate 40 mg/kg IV given 5 min before extubation is a simple, safe, cheap and effective method for blunting cardiovascular responses to tracheal extubation in ASA grade I & II patients undergoing surgery under general anesthesia. Further studies using longer post-operation follow-ups can focus the advantage, other beneficial effects, and safety of MgSo4 when used for the purpose of attenuating the hemodynamic changes associated with extubation in patients with CAD and cerebrovascular disease, gestational HT etc.
Source of Funding
No funding sources.
Conflict of Interest
There are no conflicts of interest.
Ethical Approval
The study was approved by the Institutional Ethics Committee.
Acknowledgement
The authors thank nursing and paramedical staff associated with operation theatre and postanesthesia care unit. Special thanks to surgical departments for their co-operation for smooth conduct of the study. Lastly thanks to the patients and their relatives for providing us the platform to conduct the clinical work.
References
- Vachhani R, Gulati R. Comparative study to evaluate effects of nitroglycerine and esmolol on hemodynamic parameters in controlled hypertensive patients during emergence from anaesthesia and extubation. Int J Med Res Prof. 2017;3(3):90-3. [Google Scholar]
- Hartley M, Vaughan RS. Problems associated with tracheal extubation. Br J Anaesth. 1993;71(4):561-8. [Google Scholar]
- Tandon N, Goyal S. Comparison of dexmedetomidine and magnesium sulphate in attenuation of airway and pressor responses during extubation in patients undergoing craniotomies. Int J Contemp Med Res. 2017;4(5):1033-7. [Google Scholar]
- Nishina K, Mikawa K, Maekawa N, Obara H. Attenuation of cardiovascular responses to tracheal extubation with diltiazem. Anesth Analg. 1995;80(6):1217-22. [Google Scholar]
- Miller KA, Harkin CP, Bailey PL. Postoperative tracheal extubation. Anesth Analg. 1995;80(1):149-72. [Google Scholar]
- Lowrie A, Johnston PL, Fell D, Robinson SL. Cardiovascular and plasma catecholamine responses at tracheal extubation. Br J Anaesth. 1992;68(3):261-3. [Google Scholar]
- Malde A, Sarode V. Attenuation of the hemodynamic response to endotracheal intubation: fentanyl versus lignocaine. Internet J Anesthesiol. 2006;12(1). [Google Scholar]
- Dyson A, Isaac PA, Pennant JH, Giesecke AH, Lipton JM. Esmolol Attenuates Cardiovascular Responses to Extubation. Anesth Analg. 1990;71(6):675-8. [Google Scholar]
- Karmarkar S, Varshney S. Tracheal extubation. Contin Educ Anaesth Crit Care Pain. 2008;8(6):214-20. [Google Scholar]
- Arar C, Colak A, Alagol A, Uzer SS, Ege T, Turan N. The use of esmolol and magnesium to prevent haemodynamic responses to extubation after coronary artery grafting. Eur J Anaesthesiol. 2007;24(10):826-31. [Google Scholar]
- Chhabra B, Malhotra N, Bhardwaj M, Goel GK. Haemodynamic response to extubation: attenuation with propofol, lignocaine and esmolol. J Anaesth Clin-Pharmacol. 2003;19(3):283-8. [Google Scholar]
- Li L, Liu X, Herr K. Postoperative Pain Intensity Assessment: A Comparison of Four Scales in Chinese Adults. Pain Med. 2007;8(3):223-34. [Google Scholar]
- Guha S, Verma A. Evaluation of postoperative pain (torment) using numerical and visual analogue scales. Int J Contemp Med Res. 2017;4(5):1182-7. [Google Scholar]
- Tomlinson S. Usefulness of Rate Pressure Product (RPP) for cardiac rehabilitation exercise prescription. Intern Res Open J. ;2017(2):1-4. [Google Scholar]
- Aphale S, Singh A, Bhosale J. Comparison of diltiazem and esmolol in attenuating the cardiovascular responses to tracheal extubation. Innov J Med Health Sci. 2015;5(1):1-5. [Google Scholar]
- Shin HJ, Do SH. Magnesium sulfate: A versatile anesthetic adjuvant. Anaesth Inten Care Med. 2017;4(5). [Google Scholar]
- Sunil R, Vijay S, Jerry P. The role of intravenous magnesium sulphate in attenuating pressor response to laryngoscopy and intubation in patients undergoing major head and neck surgeries. Ain-Shams J Anesthesiol. 2014;7(3):451-5. [Google Scholar]
- Fawcett WJ, Haxby EJ, Male DA. Magnesium: physiology and pharmacology. Br J Anaesth. 1999;83(2):302-20. [Google Scholar]
- Barbosa FT, Barbosa LT, Jucá MJ, Cunha RMd. Applications of magnesium sulfate in obstetrics and anesthesia. Rev Bras Anestesiol. 2010;60(1):104-10. [Google Scholar]
- Gupta R, Mahajan L, Kaur M, Aujla K, Singh A, Kaur A. Attenuation of the pressor responses to laryngoscopy and endotracheal intubation with intravenous dexmedetomidine versus magnesium sulphate under bispectral index-controlled anaesthesia: A placebo-controlled prospective randomised trial. Indian J Anaesth. 2018;62(5):337-43. [Google Scholar]
- Lee DH, Kwon IC. Magnesium sulphate has beneficial effects as an adjuvant during general anaesthesia for caesarean section. Br J Anaesth. 2009;103(6):861-6. [Google Scholar]
- Nooraei N, Dehkordi ME, Radpay B, Teimoorian H, Mohajerani SA. Effects of intravenous magnesium sulfate and lidocaine on hemodynamic variables following direct laryngoscopy and intubation in elective surgery patients. Tanaffos. 2013;12(1):57-63. [Google Scholar]
- Zarif P, Mahmoud AAA, Abdelhaq MM, Mikhail HMS, Farag A. Dexmedetomidine versus Magnesium Sulfate as Adjunct during Anesthesia for Laparoscopic Colectomy. Anesthesiol Res Pract. 2016;2016. [Google Scholar] [Crossref]
- James MFM. I-magnesium: an emerging drug in anaesthesia. Br J Anaesth. 2009;103(4):465-7. [Google Scholar]
- Dar SA, Gupta DD. Effect of Magnesium Sulphate on Attenuation of Hemodynamic Stress Responses during Laparoscopic Abdominal Surgeries. . J Anesth Clin Res. 2015;06(12). [Google Scholar]
- Seyhan T, Tugrul M, Sungur M, Kayacan S, Telci L, Pembeci K. Effects of three different dose regimens of magnesium on propofol requirements, haemodynamic variables and postoperative pain relief in gynaecological surgery. Br J Anaesth. 2006;96(2):247-52. [Google Scholar]
- Tramer MR, Schneider J, Marti R, Rifat K. Role of Magnesium Sulfate in Postoperative Analgesia. Anesthesiol. 1996;84(2):340-7. [Google Scholar]
- Surana A. Role of magnesium-A stepahead in anaesthesia. J Anesth Crit care. 2016;6(1). [Google Scholar] [Crossref]
- Shin YH, Choi SJ, Jeong HY, Kim MH. Evaluation of dose effects of magnesium sulfate on rocuronium injection pain and hemodynamic changes by laryngoscopy and endotracheal intubation. Korean J Anesthesiol. 2011;60(5):329-33. [Google Scholar]
- Hans GA, Bosenge B, Bonhomme VL, Brichant JF, Venneman IM, Hans PC. Intravenous magnesium re-establishes neuromuscular block after spontaneous recovery from an intubating dose of rocuronium. Eur J Anaesthesiol. 2012;29(2):95-9. [Google Scholar]
How to Cite This Article
Vancouver
Agrawal CG, Khadke SJ. Comparison of IV magnesium sulphate and IV esmolol in attenuating hemodynamic extubation response after general anesthesia [Internet]. Indian J Clin Anaesth. 2020 [cited 2025 Sep 22];7(3):457-465. Available from: https://doi.org/10.18231/j.ijca.2020.083
APA
Agrawal, C. G., Khadke, S. J. (2020). Comparison of IV magnesium sulphate and IV esmolol in attenuating hemodynamic extubation response after general anesthesia. Indian J Clin Anaesth, 7(3), 457-465. https://doi.org/10.18231/j.ijca.2020.083
MLA
Agrawal, Chetan Gopal, Khadke, Suchita Joshi. "Comparison of IV magnesium sulphate and IV esmolol in attenuating hemodynamic extubation response after general anesthesia." Indian J Clin Anaesth, vol. 7, no. 3, 2020, pp. 457-465. https://doi.org/10.18231/j.ijca.2020.083
Chicago
Agrawal, C. G., Khadke, S. J.. "Comparison of IV magnesium sulphate and IV esmolol in attenuating hemodynamic extubation response after general anesthesia." Indian J Clin Anaesth 7, no. 3 (2020): 457-465. https://doi.org/10.18231/j.ijca.2020.083
