- Visibility 74 Views
- Downloads 10 Downloads
- DOI 10.18231/j.ijca.2025.002
-
CrossMark
- Citation
Efficacy of palonosetron in comparison with other 5-HT3 antagonist drugs in preventing postoperative nausea and vomiting after otorhinolaryngological surgeries: A meta-analysis
Introduction
Postoperative nausea and vomiting (PONV) is found to appear frequently and most unpleasantly in patients who have undergone otorhinolaryngological surgery and general anaesthesia. Besides postoperative pain, the adverse outcome of otorhinolaryngological surgery, PONV appears in 20–30 per cent of patients.[1] Moreover, from the patient’s perspective, the anesthetist may be seen as accountable for the sensations experienced during the early postoperative period.[2] ([Figure 1]) presents a general illustration of postoperative nausea and vomiting (PONV). Despite multiple clinical studies aimed at addressing PONV, effective prevention methods continue to be a challenge. As per the reports published in 2020 by Gan et as guidelines for the management of postoperative nausea and vomiting state that risk factors for PONV in adults such as history of PONV or motion sickness, female sex, non-smoking, younger age, general versus regional anaesthesia, use of volatile anaesthetics and nitrous oxide, postoperative opioids, duration of anaesthesia, type of surgeries.[3]
The second-generation 5-hydroxytryptamine 3 (5-HT3) receptor antagonists, neurokinin 1(NK1) receptor antagonists, and dopamine antagonists are kind of drugs used to treat nausea and vomiting. Reports on managing PONV with the clinical usage of serotonin (5-HT3) receptor antagonists a new antiemetic drug class with enhanced efficacy, lengthened action and minor aftereffects were found. 5-HT3 receptors are known to be positioned on the terminals of the vagus of the periphery and centrally in the chemoreceptor trigger zone which is the zone of the post rema of the medulla oblongata.[4]
The first serotonin antagonist named Ondansetron invention was a pivotal step in the prohibition of nausea and vomiting. Even though it is found to be more effective the reports are there on its fewer side effects when compared with other types of antiemetics.[5] The other drug Palonosetron received FDA approval in March 2008 for PONV during the period up to 24h, and 48h after surgery and was reported as a selective serotonin subtype 3 receptors (5-HT3) antagonist having a robust binding affinity used to prevent vomiting and nausea caused by chemotherapy.[6], [7] Palonosetron binds tightly with the 5-HT3 receptor in the classical neurotransmitter site at the subunit/subunit interface. The palonosetron contains two moieties called the azabicyclo ring that deeply penetrates into the binding pocket and the isoquinoline ring that sits at the cavity of the surface of the complementary subunit, capped by loop C of the principal subunit. The aromatic cage formed of four aromatic residues (W156, Y126, Y207 and W63) wraps around the azabicyclo ring tightly and mutations in the residues show no response to serotonin or show less inhibition effect by palonosetro.[8] The prophylactic administration of tropisetron is effective in preventing the incidence of PON and PONV,[9] similarly a single dose of prophylactic dexamethasone was found to significantly reduce the mean pain score in patients undergoing thyroidectomy.[10]
A recent retrospective cohort study on PONV, post-discharge nausea and vomiting (PDNV) following ambulatory eye, head, and neck surgeries revealed that 17.8% of all patients developed nausea/ vomiting (PONV/PDNV). PONV-experienced patients had a 2.79 times greater risk of reporting PDNV and the binary logistic regression found that younger age, opioid usage, and female sex were associated with an increased likelihood of experiencing any nausea and vomiting.[11] As per reports, the general incidence of PONV is aligned in the range of 20-30% while it increases up to 30-70% after ear, nose, and throat (ENT) surgeries and a few of the additional risk factors are tobacco addiction, gastroparesis, obesity, postoperative analgesic use of opioids.[12], [13], [14], [15], [16]

The researchers reported comparison studies on the efficiency of palonosetron with other drugs in the prevention of PONV of various surgeries but the results were varying and in many reports the outcome results were conflicting.
Currently, there is a notable absence of meta-analyses or systematic reviews within scientific databases that specifically compare the efficiency and efficacy of palonosetron to other pharmacological agents in the prevention of postoperative nausea and vomiting (PONV) in the context of otorhinolaryngological surgeries. This gap in the literature underscores the need for comprehensive evaluations of palonosetron's performance relative to alternative medications commonly used for PONV management. To address this issue, we set out to conduct a systematic comparison of the efficacy of palonosetron against various other antiemetic drugs in the context of preventing PONV among patients undergoing otorhinolaryngological procedures. Our analysis aims to provide valuable insights into the relative effectiveness of palonosetron compared to other therapeutic options. In addition to assessing efficacy, this study will also explore the safety profile of palonosetron relative to other medications utilized for PONV. By examining both the effectiveness and safety of palonosetron in preventing PONV, we aim to offer healthcare practitioners evidence-based recommendations that could improve postoperative care for patients undergoing otorhinolaryngological surgeries.
Material and Methods
The PRISMA (Preferred Reporting Information Standards for Meta-Analysis Research) guidelines were followed for content for Systemic Reviews and Meta-analysis.
Literature search
In the present investigation, we conducted a systematic review of the literature to identify all pertinent studies that align with our stated objectives and eligibility requirements. This review encompassed a comprehensive search of multiple databases, including PubMed, Science Direct, Embase, Scopus, and Google Scholar, up to December 2023. The specific keywords utilized in our study were "palanosteron," "post-operative nausea and vomiting," and "otorhinolaryngological surgeries." Through our thorough search process, we aimed to gather a diverse range of literature related to the efficacy and safety of palanosteron in the context of post-operative nausea and vomiting (PONV) specifically after otorhinolaryngological surgeries. Furthermore, our references include both the titles and full texts of studies that focus on the treatment of PONV, as well as comparative analyses of palanosteron with other pharmacological interventions in various global settings.
Eligibility criteria
To identify relevant studies examining the association between palonosetron and other medications for the prevention of postoperative nausea and vomiting (PONV), we systematically evaluated the titles and abstracts of numerous research articles. Only peer-reviewed articles published in English were included, while those in foreign languages were excluded. The eligibility criteria stipulated that studies must focus on drug interventions related to otorhinolaryngological surgeries and examine PONV treatment during two specific time frames: Early (0-24 hours) and Delayed (24-48 hours). Additionally, the studies had to report on the incidence of vomiting. We excluded any studies that had been conducted but not published in English, as well as those lacking information on palonosetron or relevant otorhinolaryngological surgeries. Furthermore, studies that did not present original research data were also omitted from consideration.
Extraction of data
Authors (AAA and BBB) independently retrieved all the related data from the included studies using a pre-defined, standardised data collection form and cross-checked the findings. Any disagreements between the investigators were resolved by reaching a consensus via discussion. The spreadsheet for data extraction included the following items: 1) Title; 2) name of the author; 3) year of publication; 4) study design; 5) risk of bias; 6) number of patients included; 7) duration of the drugs; 8) inclusion criteria; 9) exclusion criteria; 10) Type of drug; 11) Type of surgery; 12) dose of the drug etc.
The data were first extracted from tables or text. The corresponding authors of studies with incomplete or missing data were contacted to obtain the necessary information.
Quality of study using risk bias
Risk bias assessment is a critical step in meta-analysis because it helps evaluate the methodological quality and potential biases of the included studies. In the present study, the assessment of risk bias was conducted using the Cochrane Risk of Bias 2 (ROB2) tool software. [17], [18]
Meta-analysis
In the present study, the statistical analysis was analysed using REVMAN 5.4 software. A fixed and random effect model was computed using Review Manager. The funnel plot was not feasible because there were only nine studies for palonosetron drugs. The I² statistic was employed to evaluate the degree of heterogeneity between trials, offering insight into the variability of results across studies. Values below 40% were deemed insignificant, reflecting minimal differences between study outcomes. I² values between 40% and 60% indicated moderate heterogeneity, where some variability existed but was not excessive. High heterogeneity, marked by I² values between 60% and 90%, suggested considerable differences between trials, potentially influenced by varying study designs, populations, or interventions. Results were conveyed in the MH pooled odds ratio (for PONV) with a 95% confidence interval (CI). The P value < 0.05 was contemplated statistically significant. The number needed to treat (NNT) for PONV incidence was determined for the pooled results. Wherever heterogeneity was found to be greater than 40%, results from “random effect modelling” were reported.
Discussion
Literature search and study selection
A total of 348 scientific papers were discovered as a result of the primary search that was conducted using multiple scientific databases. The study was omitted from 130 repetitions, 10 titles with inappropriate information, and 8 papers which were not published in English. A total of 200 papers were thoroughly assessed for their abstracts; 30 of these papers were found to be related to the review subject at hand. 8 of these articles were found to meet the criteria for inclusion and were used in the analysis; the other 22 were eliminated due to insufficient data[19], [20], [21], [22], [23], [24], [25], [26] ([Figure 2]).

Study characteristics
The given ([Table 1]) showing the characteristics of the included studies.
|
S.No |
Source |
No. of cases (Total) |
Groups |
Age (yrs) |
Sex (M/F) |
Weight (kg) |
Height (cm) |
ASA - PS |
Surgery duration (hrs) |
Duration of anesthesia (hrs) |
Type of surgery |
|
1. |
Ahmed M. Abd El-Hamid et al. 2014. [19] |
30 (60) |
O |
33.93 ± 7.93 |
13/17 |
73.87 ± 13.89 |
- |
I - II |
107.7 ± 11.8* |
- |
Tympanoplasty Mastoidectomy |
|
P |
34.16 ± 6.24 |
15/15 |
75.03 ± 12.09 |
- |
109.6 ± 16.91* |
- |
|||||
|
2. |
Ahmet Aydin et al. 2019. [20] |
55 (165) |
O |
33.1±12.3 |
30/25 |
74.7±12.1 |
169.5±8.0 |
I - II |
- |
- |
middle ear surgery |
|
T |
34.3±11.8 |
30/25 |
73.2±12.7 |
167.4±8.5 |
- |
- |
|||||
|
P |
33.4±12.3 |
29/26 |
70.3±13.8 |
167.0±8.3 |
- |
- |
|||||
|
3. |
Anju Annie Paul et al. 2018. [21] |
50 (100) |
P |
30.26 |
37/13 |
61.74 |
164.20 |
I - II |
- |
3.430 |
Middle ear surgery |
|
D |
32.28 |
24/26 |
62.26 |
164.10 |
- |
3.000 |
|||||
|
4. |
Kanhaiya Kumar et al. 2022. [22] |
50 (100) |
P |
28.1±10.37 |
- |
- |
- |
I - II |
1.20±0.01 |
1.354±0.05 |
Middle ear surgery |
|
PD |
29.4± 10.24 |
- |
- |
- |
1.25±0.02 |
1.300±0.04 |
|||||
|
5. |
Mohamed I. Elahl et al. 2014. [23] |
31 (62) |
P |
35.1±8.8 |
20/11 |
- |
- |
I - II |
123±37* |
|
Middle ear surgery |
|
C |
34.9±8.8 |
18/13 |
- |
- |
129±41* |
|
|||||
|
6. |
Neeru Sahni et al. 2020. [24] |
45 (90) |
P |
34.62 ± 14.71 |
26/19 |
62.98 ± 11.60 |
161.07 ± 5.82 |
I - II |
119.44 ± 14.35* |
132.89 ± 14.86* |
Mastoidectomy Tympanoplasty |
|
PD |
31.36 ± 11.83 |
25/20 |
60.47 ± 7.66 |
161.51 ± 5.00 |
120.89 ± 15.56* |
133.11 ± 16.07* |
|||||
|
7. |
Shubhangi Sharma et al. 2019. [25] |
50 (100) |
P |
- |
- |
- |
- |
I - II |
- |
- |
Middle ear surgery |
|
O |
- |
- |
- |
- |
- |
- |
|||||
|
8. |
Vinit Kumar Srivastava et al. 2020 |
32 (64) |
P |
37.41 ± 13.17 |
18/14 |
53.97 ± 7.93 |
- |
I - II |
74.53 ± 18.64* |
- |
Timpanopasty Mastoidectomy |
|
O |
38.84 ± 12.26 |
15/17 |
55.28 ± 8.44 |
- |
79.22 ± 19.18* |
- |
Risk of bias
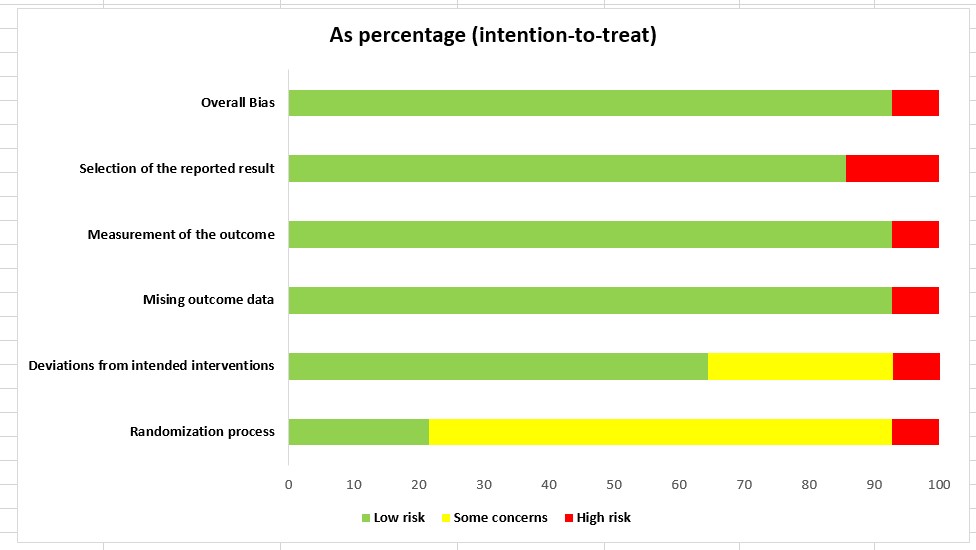
([Figure 3]) shows the findings of the risk of bias assessment conducted using the Cochrane tool for the included studies. The risk bias was assessed using Rob2 tool. In the graph, each row represents a study and each column represents one type of bias. The colour represents the reviewer’s conclusion about the risk of each type of bias in each study. Red means a high risk of bias, yellow means an unclear risk of bias, and green means a low risk of bias. The overall risk of bias results showed most studies have low risk indicating that there are no significant concerns regarding the reliability and validity of the results in the present systematic review and meta-analysis.

Quantitative meta-analysis
Postoperative nausea and vomiting (PONV) is a significant concern for patient care, ranking as the second most common issue after pain. A pivotal 1992 review by Watcha and White introduced the widespread use of the term PONV, which later became an official medical subject heading in the National Library of Medicine in 1999. [27] Proactively addressing PONV enhances patient outcomes, despite the fact that it often resolves without intervention. However, PONV can occasionally cause serious complications, such as aspiration of gastric contents, suture disruption, esophageal rupture, subcutaneous emphysema, or pneumothorax. Additionally, untreated PONV can extend a patient's hospital stay. For patients at high risk of developing PONV, the recommended approach involves administering a combination of antiemetic drugs with different mechanisms of action. This approach provides better prophylaxis and reduces drug side effects compared to single therapies. In this study, a combination of 5-HT3 antagonists, including ondansetron, palonosetron, and dexamethasone, was used, building on previous research demonstrating the superior efficacy of such combinations. Specifically, co-treatment with dexamethasone and ondansetron proved more effective than the single use of ramosetron in preventing PONV in patients undergoing middle ear surgeries.[28]
Further research, Chatterjee and colleagues reported that using palonosetron and dexamethasone together reduced the incidence of PONV from 56% to 23%, compared to single-drug therapy.[29] These findings strongly support the use of dexamethasone in combination with 5-HT3 receptor antagonists as an integral part of PONV prophylaxis, particularly in high-risk patients.
For this study, we have selected 8 published articles for analyzing the “comparison of efficacy” of Palonosetron with other similar drugs for preventing “Post Operative Nausea and Vomiting” (PONV) after otorhinolaryngological surgeries.
Group A was the treatment of palonosetron whereas Group B was treated with different drugs such as Ondansetron, Dexamethasone, and Tropisteron. In the present study, the results of PONV incidences showed significantly lower. Similarly, the results of PONV in Sharma et al. and Kumar et al. showed higher incidences in studies such as. [21], [22], [23]
In this investigation, the incidence of PONV of all the drugs post otorhinolaryngological surgeries is exhibited in ([Figure 3]). The fixed effect model was used in this study when the heterogeneity was <50% and the >50% heterogeneity was used for the random effect model.
Comprehensively, investigations of this study disclosed insignificant variations when compared to palonosetron with other drugs (p = 0.18) with a pooled estimate of 0.53 and positive confidence intervals for both the lower and upper confidence ranges (0.21, 1.35 respectively) ([Table 2] and [Figure 5]).
|
S. No. |
Study or Subgroup |
Experimental |
Control |
Weight |
Odds Ratio M-H, Random, 95% CI |
||
|
Events |
Total |
Events |
Total |
||||
|
1. |
Ahmed M. Abd El-Hamid et al. 2014. [19] |
2 |
28 |
7 |
22 |
9.7% |
0.16 [0.03, 0.90] |
|
2. |
Ahmet Aydin et al. 2019.[20] |
5 |
55 |
15 |
55 |
11.9% |
0.27 [0.09, 0.80] |
|
3. |
Ahmet Aydin et al. 2019_2.[20] |
5 |
55 |
7 |
55 |
11.5% |
0.69 [0.20, 2.31] |
|
4. |
Anju Annie Paul et al. 2018.[21] |
10 |
50 |
25 |
50 |
12.6% |
0.25 [0.10, 0.61] |
|
5. |
Kanhaiya Kumar et al. 2022.[22] |
30 |
50 |
13 |
50 |
12.7% |
4.27 [1.83, 9.97] |
|
6. |
Mohamed et al. 2013.[23] |
1 |
31 |
11 |
31 |
8.2% |
0.06 [0.01, 0.51] |
|
7. |
Neeru Sahni et al. 2022.[24] |
10 |
45 |
5 |
45 |
11.6% |
2.29 [0.71,7.33] |
|
8. |
Subhangi Sharma et al. 2019.[25] |
46 |
50 |
44 |
50 |
11.0% |
1.57 [0.41, 5.93] |
|
9. |
Vinit Kumar Srivastava et al. 2020.[26] |
3 |
32 |
12 |
32 |
10.8% |
0.17 [0.04, 0.69] |
|
Total (95% CI) |
|
396 |
|
390 |
100.0% |
0.53 [0.21, 1.35] |
|
|
Total events |
112 |
|
139 |
|
|
|
|
|
Heterogeneity: Tau2 = 1.61; Chi2 = 42.88, df = 8 (P < 0.00001); I2 = 81% |
|||||||
|
Test for overall effect: Z = 1.34 (P = 0.18) |

The outcome of an early PONV incidence exhibited a significant difference with a P value < 0.05 when compared to palonosetron with other drugs although delayed PONV response exhibited an insignificant difference with a P value > 0.05. ([Table 2])
The Forest plots representing the confidence intervals, p-values, and heterogeneity of palonosetron compared with other drugs are illustrated in [Figure 3], [Figure 4], [Figure 5], providing a visual summary of the study's results. These plots display the range of effects across various trials, offering insight into the consistency (or variability) of outcomes. Each trial's effect size is depicted as a square, with lines extending to indicate the confidence interval (CI). P-values are included to assess the statistical significance of the findings. While some heterogeneity is observed in the meta-analysis, the I² score—a statistical measure of the degree of variation between studies—indicates that this variability is notable. Heterogeneity in meta-analyses can arise from several factors, including differences in study timeframes, variations in treatments administered, experimental design discrepancies, sample sizes, and the ethnic backgrounds of the populations studied. These factors contribute to variability in outcomes, which is common in clinical trials that aggregate results from diverse settings. Addressing heterogeneity is crucial, as it can influence the interpretation of the meta-analysis findings and impact the overall conclusions regarding the drug's efficacy.
|
S. No. |
Study or Subgroup |
Experimental |
Control |
Weight |
Odds Ratio M-H, Random, 95% CI |
||
|
Events |
Total |
Events |
Total |
||||
|
1. |
Ahmed M. Abd El-Hamid et al. 2014.[19] |
2 |
30 |
5 |
30 |
12.3% |
0.36 [0.06, 2.01] |
|
2. |
Ahmet Aydin et al. 2019. [20] |
13 |
55 |
27 |
55 |
15.9% |
0.32 [0.14, 0.73] |
|
3. |
Anju Annie Paul et al. 2018.[21] |
25 |
50 |
10 |
50 |
15.6% |
4.00 [1.65, 9.72] |
|
4. |
Kanhaiya Kumar et al. 2022.[22] |
16 |
50 |
5 |
50 |
14.9% |
4.24 [1.41, 12.70] |
|
5. |
Mohamed et al. 2013.[23] |
4 |
31 |
20 |
31 |
14.1% |
0.08 [0.02, 0.29] |
|
6. |
Neeru Sahni et al. 2020.[24] |
3 |
45 |
2 |
45 |
11.8% |
1.54 [0.24, 9.66] |
|
7. |
Subhangi Sharma et al. 2019.[25] |
42 |
50 |
35 |
50 |
15.4% |
1.95 [0.76, 5.01] |
|
Total (95% CI) |
|
311 |
|
311 |
100.0% |
0.93 [0.30, 2.85] |
|
|
Total events |
104 |
|
104 |
|
|
|
|
|
Heterogeneity: Tau2 = 1.90; Chi2 = 41.66, df = 6 (P < 0.00001); I2 = 86% |
|||||||
|
Test for overall effect: Z = 0.13 (P = 0.89) |


The research reports on the palonosetron and its comparison with other medicines in preventing PONV after various surgeries were less. Preet Mohinder Singh et al. reported that Palonosetron treatment was found to be more statistically significant than placebo in preventing vomiting and nausea in the early and delayed stages in patients who have undergone elective surgery with general anaesthesia.
Palonosetron performed better than ramosetron in all three measures during the delayed phase, but none of these variables showed statistical significance during the early phase. Palonosetron showed exceptional vomiting inhibition (VI) and complete response (CR) than granisetron in the initial stage but in the delayed phase palonosetron showed no statistical significance in variables other than CR which are higher effective for Palonosetron.
In investigating the incidence of PONV associated with Palonosetron in comparison with various other medications in the course of elective surgeries performed under general anaesthesia, Xiong et al. 2015, reported that Palonosetron was more effective than ondansetron in suppressing early postoperative nausea (PON) by 49%, late PON by 47%, and late postoperative vomiting (POV) by 59%. However, no significant difference was observed in diminishing early POV between the two drugs.[30] Preet et al. 2016 reported a meta-analysiswith 22 trials on Palanosetron, and compared with ondansetron drug. The results showed that Palanosetron showed better improvement than ondansetron in all groups.[31] Kim et al. 2017 reported the meta-analysis of preventive of PONV using Palanosetron and compared with Ramosetron. The results revealed no definite difference in PONV prevention between the two drugs that are tested.[32] Eun Jin et al. 2016 reported that there are no distinguishable variations in the efficacy of Palonosetron and Ramosetron in the prevention of PON, POV, and PONV when compared with other medications during elective surgeries performed under general anaesthesia. An administration of 5-HT3 receptor antagonist during the early portion of the operation, Palonosetron showed higher efficacy than Ramosetron.[33] Nevertheless, when administered towards the conclusion of the surgical procedure, ramosetron had superior efficacy compared to palonosetron. No discernible disparities in headache or dizziness were seen between those who received palonosetron and those who received ramosetron.
Palonosetron is unique among 5-HT3 antagonists due to its high receptor binding affinity and prolonged half-life, which enables it to provide long-lasting protection against both acute and delayed phases of chemotherapy-induced nausea and vomiting (CINV). Unlike earlier 5-HT3 antagonists, such as ondansetron and granisetron, palonosetron is highly selective for the 5-HT3 receptors and demonstrates a different pharmacodynamic profile. It exhibits allosteric inhibition, leading to a change in the receptor’s conformation and increased internalization, which reduces receptor availability and enhances its antiemetic effect. Palonosetron has minimal interaction with other neurotransmitter receptors, which contributes to its relatively low incidence of side effects, such as headache or constipation. It is well-absorbed following intravenous administration, reaching peak plasma concentrations rapidly, with a volume of distribution indicative of extensive tissue distribution. Palonosetron is primarily metabolized by the CYP2D6 enzyme, with additional contributions from CYP3A4 and CYP1A2 pathways, and is eliminated through both renal and hepatic routes. Its long duration of action makes it particularly effective in preventing delayed nausea and vomiting, a significant advantage in managing patients undergoing highly emetogenic chemotherapy.
Palonosetron’s clinical implications in PONV management go beyond its pharmacokinetic profile. Compared to first-generation 5-HT3 antagonists like ondansetron and dolasetron, palonosetron demonstrates superior efficacy, particularly in preventing delayed PONV, which typically occurs more than 24 hours post-surgery. Its unique allosteric binding and positive cooperativity with the 5-HT3 receptor may contribute to this enhanced and sustained efficacy. Furthermore, studies have shown that palonosetron may reduce the overall incidence of nausea, which is often more challenging to control than vomiting, thus improving patient comfort and satisfaction. Additionally, palonosetron is often compared with other classes of antiemetics, such as neurokinin-1 (NK1) receptor antagonists (e.g., aprepitant) and corticosteroids (e.g., dexamethasone). While NK1 receptor antagonists are also effective in managing both acute and delayed PONV, palonosetron’s side effect profile tends to be more favorable, with fewer reports of headache, constipation, and fatigue. Its minimal drug interactions also make it a safer option for polypharmacy patients. When used in combination with other antiemetics like dexamethasone, palonosetron has been shown to provide synergistic effects, further reducing the incidence of PONV compared to monotherapy with older 5-HT3 receptor antagonists. Despite these advantages, its cost remains a limiting factor, especially in resource-constrained settings, where cheaper alternatives may still be prioritized.
The efficacy of Palonosetron in comparison to other 5-HT3 antagonists for PONV after otorhinolaryngological surgeries can be influenced by several factors. Patient characteristics such as age, gender, BMI, and history of PONV or motion sickness are critical, as they affect susceptibility to PONV. The type of surgery (e.g., sinus surgery or tonsillectomy) and the anesthetic technique, particularly the use of general anesthesia and opioids, also play significant roles. Drug dosage, timing of administration, and the length of observation post-surgery impact the efficacy comparison, especially since Palonosetron has a longer half-life than other 5-HT3 antagonists. Study design factors such as randomization, blinding, and study size can introduce bias, while pharmacological differences between the drugs, including receptor affinity and side effect profiles, also contribute.
Limitations
While this meta-analysis highlights the effectiveness of palonosetron in preventing postoperative nausea and vomiting (PONV), several limitations should be considered. First, palonosetron primarily inhibits serotonin (5-HT3) receptors, which restricts its efficacy to serotonin-related pathways of nausea and vomiting. This specificity means that palonosetron may be less effective in situations where other pathways, such as dopamine or neurokinin-1 (NK-1) receptors, play a significant role. In contrast, other antiemetic drugs like aprepitant, an NK-1 receptor antagonist, or dexamethasone, a corticosteroid, have broader mechanisms of action that may provide more comprehensive PONV prevention.
Additionally, palonosetron is primarily intended for the prevention of PONV rather than the treatment of acute symptoms. Its delayed onset of action, despite a prolonged half-life, makes it less suitable for rapid symptom relief compared to faster-acting agents like ondansetron. Moreover, existing studies suggest that palonosetron may be less effective in preventing early PONV, particularly in high-risk patients, when compared to combination therapies that target multiple receptors, such as 5-HT3 antagonists combined with dexamethasone or NK-1 antagonists.
Another limitation is the cost of palonosetron, which tends to be higher than that of other 5-HT3 antagonists. This financial consideration could influence its use in healthcare settings with budget constraints. Lastly, while palonosetron’s long half-life offers extended protection against PONV, this benefit may not be necessary in shorter surgeries, where shorter-acting agents may suffice. Consequently, despite its advantages, the role of palonosetron in PONV management may be limited in scenarios requiring broader or more immediate antiemetic coverage.
Conclusion
This study compared the effectiveness of palonosetron with other antiemetic drugs in suppressing postoperative nausea and vomiting (PONV) in otorhinolaryngological surgeries. Palonosetron demonstrated significant efficacy in preventing both early and delayed PONV compared to other medications used alone. In contrast, the combined effects of other drugs did not yield a similarly effective outcome. The extended duration of action and notable efficacy of palonosetron position it as a valuable option for patients undergoing these procedures. However, further investigations are necessary to validate these findings and to determine the optimal dosage and administration protocols tailored to specific ENT surgeries and individual patient risk factors.
Abbreviations
PONV: Postoperative nausea and vomiting; PO: Post operation; PON: Postoperative nausea; POV: Postoperative vomiting; ENT: Ear, Nose, Throat; 5-HT3 receptor: 5-hydroxytryptamine 3 Receptors (Serotonin receptors); VI: Vomiting inhibition; CR: Complete response; PRISMA: Preferred reporting information standards for meta-analysis research; FDA: Food and drug administration; CI: Confidence interval; NNT: Number needed to treat.
Source of Funding
None.
Conflict of Interest
None.
Ethical Approval
Not required.
References
- LASC Mercado, R Liu, KM Bharadwaj, JJ Johnson, R Gutierrez. Association of intraoperative opioid administration with postoperative pain and opioid use. JAMA Surg 2023. [Google Scholar]
- J Málek, P Ševčík, D Bejšovec, T Gabrhelík, M Hnilicová, I Křikava. Postoperative pain management. Prague, Czech Republic: Mladá Fronta 2017. [Google Scholar]
- TJ Gan, KG Belani, S Bergese, F Chung, P Diemunsch, AS Habib. Fourth consensus guidelines for the management of postoperative nausea and vomiting. Anesth Analg 2020. [Google Scholar]
- S Bhatt, T Devadoss, SN Manjula, J Rajangam. 5-HT3 receptor antagonism a potential therapeutic approach for the treatment of depression and other disorders. Curr Neuropharmacol 2021. [Google Scholar]
- L Philpott, E Clemensen, GT Lau. Droperidol versus ondansetron for nausea treatment within the emergency department. Emerg Med Australas 2023. [Google Scholar]
- HR Chun, IS Jeon, SY Park, SJ Lee, SH Kang, SI Kim. Efficacy of palonosetron for the prevention of postoperative nausea and vomiting: a randomized, double-blinded, placebo-controlled trial. Br J Anaesth 2014. [Google Scholar]
- SH Kim, JY Hong, WO Kim, HK Kil, MH Karm, JH Hwang. Palonosetron has superior prophylactic antiemetic efficacy compared with ondansetron or ramosetron in high-risk patients undergoing laparoscopic surgery: a prospective, randomized, double-blinded study. Korean J Anesthesiol 2013. [Google Scholar]
- E Zarkadas, H Zhang, W Cai, G Effantin, J Perot, J Neyton. The binding of palonosetron and other antiemetic drugs to the serotonin 5-ht3 receptor. Structure 2020. [Google Scholar]
- IJ Kim, GJ Choi, HJ Hwang, H Kang. Effect of prophylactic tropisetron on post-operative nausea and vomiting in patients undergoing general anesthesia: systematic review and meta-analysis with trial sequential analysis. J Pers Med 2024. [Google Scholar]
- R Ahmad, M Changeez, ATU Din, A Iftikhar, HB Ahmad, A Mujtaba. Role of prophylactic dexamethasone before thyroidectomy in reducing postoperative pain, nausea and vomiting. Cureus 2019. [Google Scholar]
- M Xiao, D Yao, KG Fields, P Sarin, AA Macias, S Eappen. Postoperative and postdischarge nausea and vomiting following ambulatory eye, head, and neck surgeries: a retrospective cohort study comparing incidence and associated factors. Perioper Med (Lond) 2024. [Google Scholar]
- CL Burlacu, D Healy, DJ Buggy, C Twomey, D Veerasingam, A Tierney. Continuous gastric decompression for postoperative nausea and vomiting after coronary revascularization surgery. Anesth Analg 2005. [Google Scholar]
- K Leslie, PS Myles, MTV Chan, MJ Paech, P Peyton, A Forbes. Risk factors for severe postoperative nausea and vomiting in a randomized trial of nitrous oxide-based vs nitrous oxide-free anaesthesia. Br J Anaesth 2008. [Google Scholar]
- LR Ferrari, JV Donlon. Metoclopramide reduces the incidence of vomiting after tonsillectomy in children. Anesth Analg 1992. [Google Scholar]
- K Erkalp, NK Erkalp, MS Sevdi, AY Korkut, H Yeter, SS Ege. Gastric decompression decreases postoperative nausea and vomiting in ent surgery. Int J Otolaryngol 2014. [Google Scholar]
- CAM Patti, JE Vieira, FEM Benseñor. Incidência e profilaxia de náuseas e vômitos na recuperação pós-anestésica de um hospital-escola terciário. Rev Bras Anestesiol 2008. [Google Scholar] [Crossref]
- JAC Sterne, J Savović, MJ Page, RG Elbers, NS Blencowe, I Boutron. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019. [Google Scholar]
- JP Higgins, JA Sterne, J Savovic, MJ Page, A Hróbjartsson, I Boutron. A revised tool for assessing risk of bias in randomized trials. Cochrane Database Syst Rev 2016. [Google Scholar]
- A Aydin, M Kaçmaz, A Boyaci. Comparison of ondansetron, tropisetron, and palonosetron for the prevention of postoperative nausea and vomiting after middle ear surgery. Curr Ther Res Clin Exp 2019. [Google Scholar]
- N Sahni, N Panda, A Kumar, I Bala, N Panda. Comparison of palonosetron with combination of palonosetron and dexamethasone in the prevention of post operative nausea and vomiting in patients undergoing middle ear surgery: a prospective randomized trial. Indian J Otolaryngol Head Neck Surg 2022. [Google Scholar]
- S Sharma, S Khanna, J Das, Y Mehta, K Handa. A randomized study to compare palonosetron with ondansetron for prevention of postoperative nausea and vomiting following middle ear surgeries. J Anaesthesiol Clin Pharmacol 2019. [Google Scholar]
- VK Srivastava, S Khan, S Agrawal, SA Deshmukh, P Shree, PP Misra. Comparison of palonosetron-dexamethasone and ondansetron-dexamethasone for prevention of postoperative nausea and vomiting in middle ear surgery: a randomized clinical trial. Braz J Anesthesiol 2020. [Google Scholar]
- K Kumar, R Kumar, M Hussain, B Kumari, A Kumar. Comparison of palonosetron versus palonosetron and dexamethasone for prevention of postoperative nausea and vomiting after middle ear surgeries: a randomized controlled study. Anesth Essays Res 2022. [Google Scholar]
- AA Paul, SK George, R Ranjan, M Kurien, A Mohan, LG Ninan. Randomised control study of palonosetron versus dexamethasone in preventing postoperative nausea and vomiting following ear and nose surgeries under general anesthesia. J Clin Diagn Res 2018. [Google Scholar]
- AM AbdEl-Hamid, MS Othman, EE Afifi. Palonosetron versus ondansetron for prevention of postoperative nausea and vomiting during middle ear surgery: a double-blind, randomized, comparative trial.. Ain-Shams J Anesthesiol 2014. [Google Scholar]
- MI Elahl, M Badea. Palonosetron in preventing postoperative nausea and vomiting in middle ear surgery: a randomized-controlled study. Egypt J Otolaryngol 2013. [Google Scholar]
- MF Watcha, PF White. 9 Antiemetics. Clin Anaesthesiol 1995. [Google Scholar]
- S Desai, MCB Santosh, R Annigeri, V Santoshi, R Rao. Comparison of the antiemetic effect of ramosetron with the combination of dexamethasone and ondansetron in middle ear surgery: A double-blind, randomized clinical study. Saudi J Anaesth 2013. [Google Scholar]
- A Chatterjee, S Sahu, M Paul, T Singh, S Singh, P Mishra. Comparison of efficacy of palonosetron-dexamethasone combination with palonosetron or dexamethasone alone for prophylaxis against post-operative nausea and vomiting in patients undergoing laparoscopic cholecystectomy. Indian J Anaesth 2017. [Google Scholar]
- C Xiong, G Liu, R Ma, J Xue, A Wu. Efficacy of palonosetron for preventing postoperative nausea and vomiting: a systematic review and meta-analysis. Can. Can J Anaesth 2015. [Google Scholar]
- PM Singh, A Borle, D Gouda, JK Makkar, MK Arora, A Trikha. Efficacy of palonosetron in postoperative nausea and vomiting (Ponv)-a meta-analysis. J Clin Anesth 2016. [Google Scholar]
- MS Kim, JH Park, YS Choi, SH Park, S Shin. Efficacy of palonosetron vs. Ramosetron for the prevention of postoperative nausea and vomiting: a meta-analysis of randomized controlled trials. Yonsei Med J 2017. [Google Scholar]
- E Ahn, G Choi, H Kang, C Baek, Y Jung, Y Woo. Palonosetron and ramosetron compared for effectiveness in preventing postoperative nausea and vomiting: a systematic review and meta-analysis. Nishimura W, editor. PLoS One 2016. [Google Scholar]
