Introduction
In recent decades, there has been a notable shift towards minimally invasive laparoscopic surgeries. Laparoscopic surgeries are performed routinely with minimal anesthesia-related complications. Capnothorax, stemming primarily from abdominal carbon dioxide (CO2) insufflation during laparoscopy, constitutes a relatively uncommon but life-threatening complication. Due to the rarity of capnothorax, it is frequently missed.1 Diagnosis hinges primarily on clinical signs such as auscultation of breath sounds, peak airway pressure, arterial oxygen saturation (SpO2), and end-tidal carbon dioxide tension (EtCO2). These indicators, however, can overlap with other respiratory complications encountered during laparoscopy, such as endobronchial migration of the endotracheal tube or pneumothorax. Consequently, diagnosing capnothorax requires meticulous attention to the sequence of events and a high level of suspicion. In this context, we present two cases where a presumptive diagnosis of capnothorax was reached during laparoscopic procedures.
Case Presentation
Case 1
A 68-year-old male (167 cm, 65 kg) underwent diagnostic laparoscopy and abdominoperineal resection for rectal malignancy. Except for a mild anaemia, patient’s other laboratory investigations were normal. He had stable vitals preoperatively. A standard induction technique was employed, and endotracheal placement of the tube was confirmed by bilateral equal air entry. Mechanical ventilator was set to deliver tidal volume (TV) of 500 ml at respiratory rate (RR) of 12/ minute in volume control ventilation mode (VCV). Anaesthesia was maintained with 50:50 mixture of oxygen: nitrous oxide (O2:N2O) and sevoflurane was titrated to achieve MAC-age of 1. Pulse oximeter showed an SpO2 of 100% with fraction of inspired oxygen (FiO2) of 0.5. Pneumoperitoneum was created by insufflating 5 L/min of CO2, to a set pressure of 12 mmHg. Following this, 20° Trendelenburg position was given. Within ten minutes of pneumoperitoneum, the EtCO2 and Peak Inspiratory Pressure (PIP) increased from 33 mmHg and 18 cmH2O to 40 mmHg and 40 cmH2O respectively. A slow drop in SpO2 to 92% and a fall in systemic arterial blood pressure from 110/70 mmHg to 80/40 mmHg was also observed. The surgeons were notified to pause laparoscopy and deflate the abdomen. Over the next few minutes, the EtCO2 and PIP improved to 36 mmHg and 36 cmH2O respectively, and the BP increased to 90/50 mmHg, though SpO2 remained at 92% with an FiO2 of 0.5. On auscultation, air entry could be appreciated in all lung areas on right side. On left side, it was absent in left infraclavicular region while it was present in the infra-axillary and infra-mammary areas. Endobronchial migration of tracheal tube was ruled out using video bronchoscopy. As hemodynamics improved with deflation of the abdomen, we decided to manage conservatively with close monitoring. N2O was cut off and 100% O2 was administered. Over the next 10 minutes, the SpO2 slowly improved to 96%, BP increased and the EtCO2 and PIP decreased. A second attempt to inflate the abdomen resulted in similar changes. Laparoscopy was abandoned and laparotomy was performed. In the next 45 minutes, the SpO2 rose to 100%, the EtCO2 dropped to 34 mmHg, the PIP dropped to 18 cmH2O, and the blood pressure likewise stabilized. Air entry was also appreciated in the left infraclavicular region. The sequence of events being triggered by pneumoperitoneum and its spontaneous resolution following deflation of the abdomen led us to diagnose it as a capnothorax.
Case 2
A 24-year-old male diagnosed with left eventration of diaphragm underwent diagnostic laparoscopy followed by thoracoscopic plication of diaphragm. After standard induction and confirmation of endotracheal intubation, ventilator was set in VCV mode to deliver TV of 500 ml at RR of 12/ minute. Anaesthesia was maintained with a O2:N2O mixture and sevoflurane titrated to attain a MAC of 1. The SpO2, Peak inspiratory pressure and EtCO2 were 100%, 14 cmH2O and 35 mmHg respectively. Pneumoperitoneum was created as described in the previous case. Following this, a 15° reverse Trendelenburg position was given. After 10 minutes of pneumoperitoneum, the EtCO2 and PIP increased to 40 mmHg and 42 cmH2O respectively, SpO2 dropped to 92%. On auscultation, air entry was normal in right lung. The left lung area could not be auscultated as it was prepped with antiseptic and sterile draped for thoracoscopy. Endobronchial migration was ruled out by flexible bronchoscopy. By then, surgeon had assessed the extent of eventration and decided to go ahead with thoracoscopy. Hence the abdomen was deflated. The SpO2, PIP and EtCO2 immediately improved to 96%, 36 cmH2O and 38 mmHg respectively. Left lung isolation was achieved with a bronchial blocker in left main bronchus. Right lateral decubitus position was used for thoracoscopy. As the thoracoscopy port was inserted, a gush of gas was heard. All monitored parameters immediately returned to baseline normal levels. Thoracoscopy did not reveal any lung injury or parenchymal pathology such as bulla that could result in a pneumothorax. Even with one-lung ventilation, the PIP and EtCO2 were not as high as that which had occurred with capnothorax. No air leak was noticed during lung expansion at the conclusion of surgery ruling out any damage to the underlying lung. Chest tube that was inserted at the conclusion of procedure did not exhibit any air leaks either. As there were no plausible causes for a pneumothorax, we concluded that the gas build up was caused by capnothorax.
After procedure, both patients were monitored in post-operative ward for four hours following extubation. On follow-up, they had stable vitals and were discharged from hospital three days later.
Figure 1
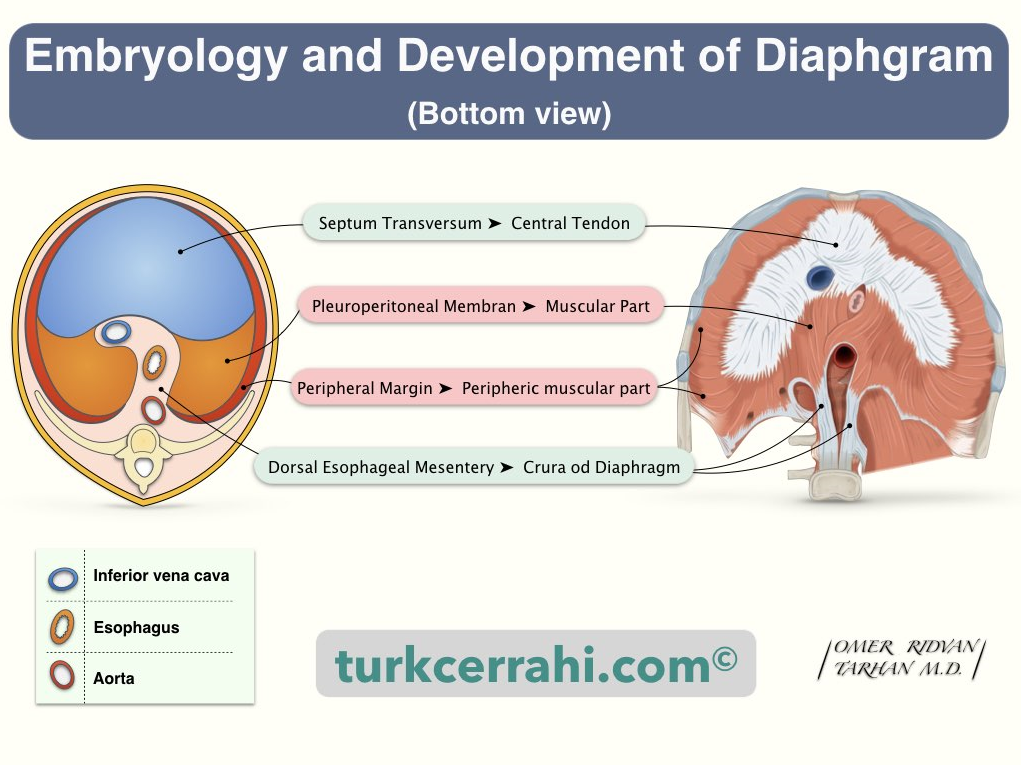
Diaphragm development and potential communicating channels for Capnothorax.2 Reproduced with permission from turkcerrahi.com
Discussion
Respiratory complications associated with laparoscopic surgeries include endobronchial migration of the tracheal tube, subcutaneous emphysema, pneumothorax, capnothorax, and air embolism. Among these, the first four can lead to an increase in Peak Inspiratory Pressure (PIP). Pneumoperitoneum-induced elevation of the diaphragm may result in the migration of the endotracheal tube into the right main bronchus, a condition easily diagnosed through auscultation or direct visualization using a flexible bronchoscope.
Distinguishing between capnothorax and pneumothorax presents a greater challenge. Capnothorax occurs when CO2 used for pneumoperitoneum enters thoracic cavity through potential pleuroperitoneal communicating channels. Weak areas in the diaphragm, including major openings for inferior vena cava (IVC), oesophagus, and aorta, are vulnerable sites (Figure 1).2 Additionally, other weak areas may exist due to the complex embryological development of diaphragm from various components such as the septum transversum, body wall, dorsal mesentery of oesophagus, and pleuroperitoneal folds.
In cases of elevated airway pressure and desaturation, an increase in End-Tidal CO2 (EtCO2) suggests capnothorax, whereas a decrease is indicative of pneumothorax.3 In both our patients, as airway pressures decreased upon abdominal deflation, a presumptive diagnosis of capnothorax rather than pneumothorax was established, and a conservative approach was adopted. Notably, nitrous oxide (N2O), being 30 times more soluble than nitrogen, readily diffuses into closed spaces, increasing volume and pressure, and should be promptly discontinued. In the event of pneumothorax, continued positive pressure ventilation may exacerbate air leakage, necessitating urgent chest tube insertion.
The first case of pneumothorax during laparoscopy was reported in 1952 by Riegel.4 In 1973, Doctor and Hussain reported the first instance of bilateral pneumothorax associated with laparoscopy.5 Clinical manifestations such as cyanosis, a tight reservoir bag, reduced bilateral air entry, tachycardia, and hypotension prompted them to obtain a chest X-ray, revealing bilateral pneumothorax. Gas analysis confirmed the presence of 20% CO2, confirming the diagnosis as capnothorax. This was treated invasively by inserting an intercostal drain tube. Despite advancements, sporadic cases of capnothorax during various laparoscopic procedures persist.6, 7 The severity of capnothorax can vary widely, ranging from small, rapidly resolving cases when promptly diagnosed to massive and potentially life-threatening bilateral occurrences, as highlighted by Doctor and Hussain.5
Since the time of Doctor and Hussain, significant technological advancements in monitoring and ventilation, such as SpO2, EtCO2, and PIP, have emerged. These innovations facilitate early detection of capnothorax even before hemodynamic compromise occurs. Moreover, there is now a better understanding that capnothorax can often resolve within 30-60 minutes with measures such as abdominal deflation, increased FiO2, and application of positive end-expiratory pressure.8 Based on available literature, we can conclude that capnothorax usually happens early, during the first 30 to 45 minutes following a pneumoperitoneum rather than later. This pattern can be attributed to gradual entry of gas into thoracic cavity through potential channels, initiated by increased intra-abdominal pressure due to capnoperitoneum.
In recent years, point-of-care ultrasound has emerged as a simple and non-invasive tool for diagnosing intrapleural gas at the bedside. The absence of lung sliding in B-mode and the appearance of a barcode sign in M-mode are considered 100% specific for detecting intrapleural gas.9, 10 Additionally, the resolution of capnothorax can also be effectively monitored using the 'lung point' sign.11
Conclusion
This case report demonstrates that Capnothorax can occur during CO2 pneumoperitoneum even without an injury to the diaphragm. A sudden increase in PIP and EtCO2 after pneumoperitoneum which is disproportionate to the increase in intra-abdominal pressure should arouse suspicion of capnothorax. Capnothorax can be managed conservatively by discontinuing laparoscopy, deflating the abdomen, stopping N2O and increasing FiO2. Spontaneous resolution occurs in 30 min to 1 hour. Our case report also demonstrates that if detected early, severe adverse consequences of a capnothorax can be averted.
