Introduction
Awake tracheal intubation using flexible fiberoptic bronchoscope (FOB) is a safe and most reliable technique for the anticipated difficult airway with an overall success rate of 99.4%.1 However, optimal topical anaesthesia is necessary to ensure patient comfort, reduce complications and improve success rates. Of late, dexmedetomidine nebulisation has been used in various outpatient procedures as an effective premedication agent.2, 3 When used for awake FOB, it has been found to provide better operating conditions with reduced coughing as compared to standard lidocaine nebulisation, nebulised lidocaine-fentanyl as well as intravenous dexmedetomidine.4 Kumar et al. reported adequate intubating conditions on using dexmedetomidine as an adjunct for AFOI in a case series of four patients.5 But there is a need to compare the efficacy of this technique with the existing techniques.
With this background information, our aim was to evaluate the efficacy of dexmedetomidine-lidocaine in comparison to lidocaine nebulisation to achieve successful intubation on basis of four-point intubation condition assessment score and compare the patient’s comfort as assessed by five-point intubation comfort scale and three-point behavior scale, hemodynamic stability, need for additional sedation, lidocaine aliquots, intubation attempts and complications.6
Materials and Methods
This observational cross-sectional study was conducted at the tertiary care teaching hospital over a period of 12 months after approval from institutional ethics committee (ECR/483/Inst/UK/2013/RR-16) and clinical trial registration (CTRI/2021/10/037069). Ninety six American society of anaesthesiology (ASA) I- III, aged 18-65 years adult patients with anticipated difficult airway scheduled for elective surgery planned for AFOI under general anaesthesia were included. Indications for AFOI included head and neck malignancies, temporomandibular joint dysfunction, and facial trauma with restricted mouth opening. Patients who refused to give consent, those with hepatic, renal disorders, conduction blocks, bleeding diathesis, pregnancy and those who were allergic to drugs used in the study were excluded from the study. After thorough preoperative assessment, AFOI procedure was explained and written informed consent was obtained from patients. Standard fasting guidelines, six hours for solids and two hours for clear liquids was advised. Patients were premedicated with ranitidine 150 mg, alprazolam 0.25 mg per oral at night and two hours prior to the surgery and glycopyrrolate 0.2 mg intramuscularly 60 minutes before procedure.
In the operating room intravenous canula was secured and Standard multi-parameter monitor (Mindray Lumec 12) was attached to record non-invasive blood pressure, heart rate, oxygen saturation and electrocardiography. Preprocedural sedation was assessed using Ramsay sedation score (RSS). Baseline values of all hemodynamic parameters were recorded.
As per the discretion of anaesthesiologist, patients were either nebulised with dexmedetomidine 1mcg/kg + 4ml of 4% lidocaine (160mg) in Group A, and 4ml of 4% plain lidocaine (160mg) in Group B.
Hudson RCI 1885 Micro Mist (California prop.65) Nebulizer with disposable Adult Nebulizer Mask and 7' Tubing was filled with drug mixture and driven by a flow of 6-8 litres/min of oxygen. After strapping the facemask over the patient’s nose and mouth, patients were instructed to breathe through their nose till the drug was completely nebulised. Post nebulisation vitals were again recorded. Two drops of xylometazoline 0.1% were instilled in each nostril. Nasal patency was checked and preferred nostril for intubation was dilated using nasopharyngeal airway 6.5-7.5mm smeared with 2% lignocaine jelly.10% lidocaine (total dose 40mg) was sprayed over the tonsillar pillars and back of throat and nose to anaesthetise the posterior nasopharynx and oropharynx. Appropriate size flexo-metallic endotracheal tube (ETT) was mounted on the flexible FOB (Olympus BF 150 IS). For males 7.0/7.5mm internal diameter armoured endotracheal tube and for female 6.5/7.0mm internal diameter armoured tube was used depending upon the nasal patency. The procedure was performed by trained consultant anaesthesiologists. The FOB was lubricated with water soluble ointment and introduced through the selected nostril into the nasopharynx with patient lying in supine position. Supplemental oxygen 2 L/min was given through nasal prongs. The epiglottis and the vocal cord were identified and if needed supplemental 10% lidocaine aliquots were given through the working port of fiberoptic scope. Maximal dose was not to exceed 9mg/kg and total dose utilised was noted.5 In case of moderate to severe cough and/or gag reflex an additional sedation in form of intermittent doses of midazolam (1mg) intravenously was given and recorded. Once the scope passed into the trachea, the ETT was threaded till 2-3 cm above the carina. Post ETT placement confirmation with side stream capnometry and auscultation, anaesthesia was induced. Endotracheal cuff was inflated, and tube was secured. Hemodynamic parameters were recorded pre nebulisation and during the procedure at an interval of 0, 3, 5 and 10 minutes heart rate, oxygen saturation and systolic, diastolic and mean arterial blood pressure were recorded. Total dose of lidocaine and sedatives (midazolam, if used) were noted. Additional details included procedural trauma, number of intubation attempts, unsuccessful intubation and adverse effects like laryngospasm, bronchospasm and local anaesthetic toxicity were also noted. The primary outcomes were noted by an independent observer not involved in the patient care or in the preparation of the nebulisation. The intubating condition assessment four-point grading scale included cough severity, vocal cord position and intubating conditions. The patient comfort score was defined using five-point fibreoptic intubation comfort score and three-point behaviour score immediately post intubation. Both scores are described in (Table 1).
Sample size calculation
The formula for calculated sample size is given below: -
n = (Zα/2+Zβ)2 * (p1(1-p1)+p2(1-p2)) / (p1-p2)2
Where,
Zα/2 = critical value of the Normal distribution at α/2 (for a confidence level of 95%, α is 0.05 and the critical value is 1.96), Zβ is the critical value of the normal distribution at β (for a power of 80%, β is 0.2 and its critical value is 0.842).
Based on a previous study Kumari P et al. p1 is the expected proportion of patients with cough score ≤ 1 (primary objective)in the lignocaine group which has been taken as 50% and p2 is the expected proportion of patients with cough score ≤ 1 (primary objective) in the lignocaine+dexmedetomidine group which has been taken as 80%.4
The sample size of 46 in each group was determined taking 1:1 ratio of proportion of intubation condition assessment score in each group. Taking the 2 -sided type 1 error of 0.05 and power of 80% the sample size of 92 patients was calculated. Taking into account drop out cases we took total sample size as 96, i.e. 48 in each group.
Statistical analysis
The sample size was calculated based on the primary outcome, cough and gag reflex score which was evaluated using Mann-Whitney U test for comparison with non-parametric data. This was based on study conducted by Kumari et al.4 Secondary outcomes were evaluated using Shapiro-Wilk Normality test and Kolmogorov-Smirnov goodness- of-fit test. Descriptive statistics were performed on all demographic data. All data were presented as medians and interquartile ranges. Categorical covariates were compared using frequency and percentages and continuous covariates were evaluated for normality using a Shapiro-wilk Normality test and Kolmogorov Smirnov goodness-of-fit test. A p-value of <0.05 was considered statistically significant. Data were analysed using Statistical package for Social Sciences (SPSS) version 22 (IBM Corp, Armonk, NY, USA).
Results
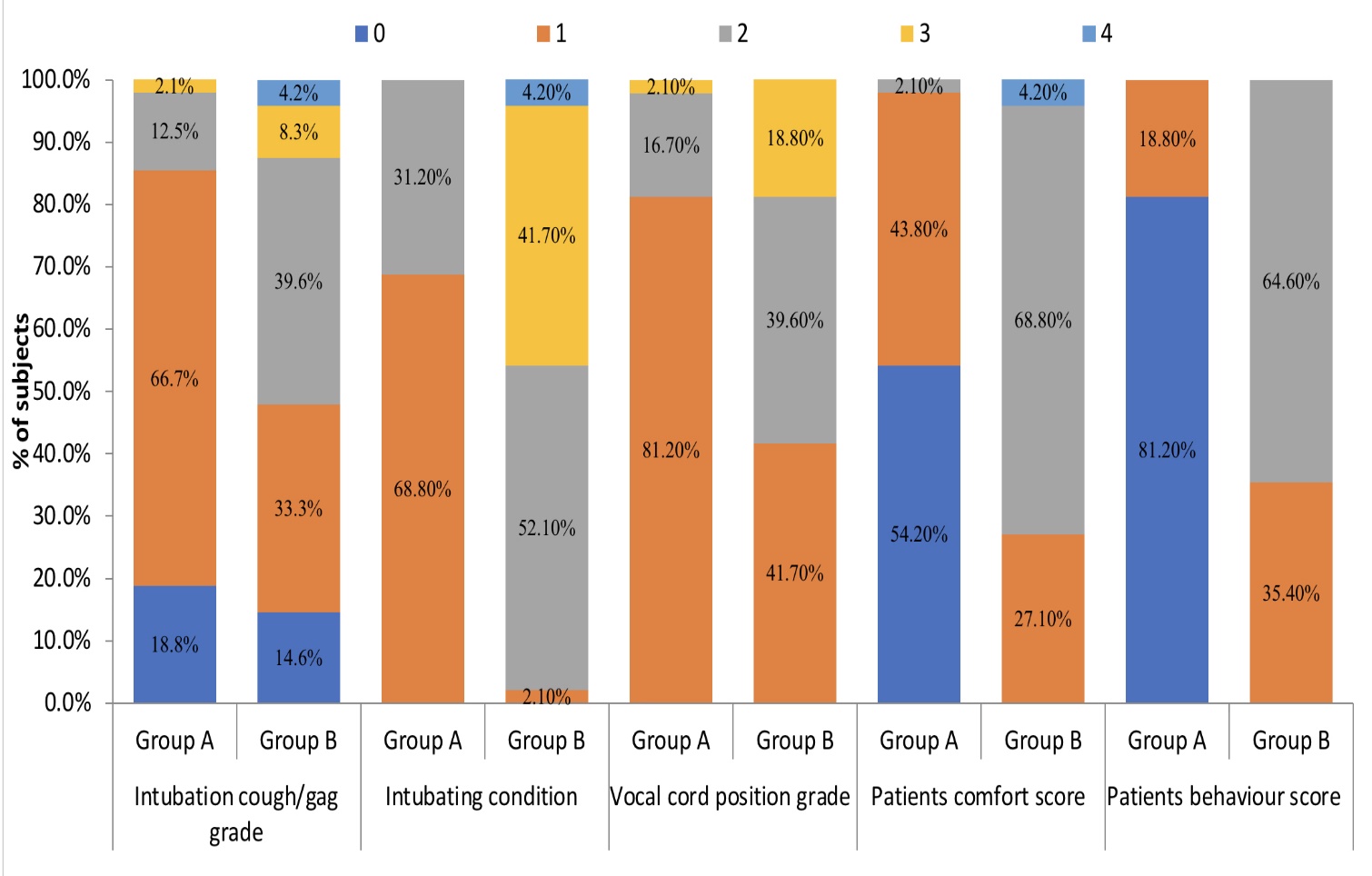
96 patients with anticipated difficult airway who underwent AFOI were included in this study. Among them 48 patients nebulised with dexmedetomidine+lidocaine and 48 with plain lidocaine. Demographic characteristics were similar in both groups (Figure 1). Comparison of airway parameters and associated variables amongst the groups (Table 2). Cough score in group A were significantly lower in comparison to group B (p<0.001). Patient behaviour as calculated by three point behaviour score was better in group A in comparison to group B (p<0001).
Patients comfort as calculated by five-point fiberoptic intubation comfort score were better in group A than group B (p<0.001) (Figure 2). Baseline hemodynamic parameters were comparable in both the groups. Hemodynamic parameters recorded at various time points indicated better stability in the dexmedetomidine group (p<0.05). Heart rate (HR) increased at all-time points in Group B when compared to group A. The maximal rise in HR from preoperative values was 10 beats/minute in the group B and 4 beats per minute in group A and maximum rise SBP was 8 mmHg from preoperative values in group B whereas it was 3 mmHg in group A (Figure 3, Figure 4). Only 3 patients in group A required sedation compared with 19 patients in group B (p<0.001) & total lidocaine consumption was higher in group B [median IQR 84 (42- 84)] than group A [median IQR 42 (21-84)] (Table 3). No difference in the oxygen saturation (SpO2) during the procedure was observed in patients in both groups. One patient in lidocaine group had severe bronchospasm needing bronchodilators and steroids. No other adverse outcomes like drug toxicity, over sedation, sore throat and hoarseness of voice was encountered in our study.
Figure 3
Hemodynamic variability comparison in both the groups
(SBP – Systolic blood pressure DBP – Diastolic blood pressure MAP –Mean blood pressure)

Figure 4
Hemodynamic variability comparison in both the groups
(HR – Heart rate SpO2- Oxygen saturation)

Table 1
Intubation grading, conditions, vocal cord position, patient comfort (Puchner) and behaviour Scales used in the study7, 8
Table 2
Comparison of airway parameters and associated variables in both groups
Table 3
Drug requirements amongst the groups.
Discussion
We designed this study to evaluate the effectiveness of dexmedetomidine - lidocaine nebulisation in patients requiring AFOI. In this study conducted on a cohort of 96 patients, we found significant attenuation of cough and gag reflex (p<0.001), with better intubating conditions (p<0.001) and preserved hemodynamics in the dexmedetomidine+lidocaine group versus plain lidocaine group. Also patients in this group had Better comfort score (p <0.001) with minimal to no requirement of supplemental sedation.
Anaesthesia for AFOI is challenging as balance has to be maintained between patient comfort and safety, different techniques in form of airway blocks, nebulisation, lozenges, gargles, and spray as you go with or without sedation have been utilised for producing favourable intubating conditions.7, 8 But to date, no single standardised technique exists to achieve awake intubation with minimal sequelae.
Patient cooperation and comfort is crucial for AFOI, thus ideal sedation should ensure good patient comfort, smooth intubating conditions, and stable hemodynamics without respiratory depression.7, 8, 9 Numerous studies have reported success with various sedative agents, including midazolam, propofol, ketamine, remifentanil and dexmedetomidine.9, 10, 11 However dexmedetomidine could be more advantageous due to its analgesic, anxiolytic, sympatholytic properties with minimal respiratory depression.12 Dexmedetomidine, an alpha-2 adrenergic receptor agonist activates post-synaptic receptors in the locus coeruleus, and induces conscious sedation by activation of endogenous sleep pathway.13 Various authors have found better patient satisfaction and endoscopic conditions with minimal hemodynamic variability post addition of intravenous dexmedetomidine for AFOI.9 But side effects like hypotension, bradycardia, nausea and atrial fibrillation have been reported with its use in the intravenous route.14
Recently, use of inhalational dexmedetomidine has been evaluated in paediatric population for premedication with good results.15 Superior sedation scores compared with oral midazolam have been reported with use of intranasal dexmedetomidine paediatric premedication.16 Also use of nebulised dexmedetomidine in paediatric premedication has led to smoother induction of GA with more rapid recovery.3, 17
Nebulisation seems to be a viable alternative route for sedation as it deposits droplets of drug directly over mucosa, bypassing the enterohepatic circulation and it is relatively safe, non-invasive and has good patient compliance. Additionally, it results in less mucosal irritation, coughing episodes, hoarseness, and nasal discomfort. When given via nebulisation, the nasal mucosa accounts for 65% of dexmedetomidine bioavailability, whereas the buccal mucosa accounts for 82% respectively.18, 19
No large studies have been conducted till date to evaluate the efficacy of dexmedetomidine via inhalational route for AFOI. A case series on four patients reported successful AFOI after dexmedetomidine nebulisation with good patient comfort.4 Considering excellent results with nebulised dexmedetomidine for various purposes, we aimed to ascertain its role for AFOI and compare it with our existing technique.
Dexmedetomidine on topical application to the airway causes smooth muscle relaxation, bronchodilatation and attenuation of cough and gag reflexes.20 Various authors have reported significant attrition in cough scores on use of dexmedetomidine-lidocaine nebulisation for awake bronchoscopy as compared to conventional techniques.2 This could also be attributed to effect of dexmedetomidine on increasing local anaesthetic action. In our study too we found significant attenuation of cough and gag reflexes in dexmedetomidine group with 41 patients experiencing no to minimal cough compared to 23 patients in lidocaine group (p<0.001).
Nebulisation with plain lidocaine has been reported to not be superior to techniques such as airway blocks in terms of vocal cord position and intubating conditions.6, 7 However, in altered airway anatomy such as head and neck cancers, blocks would be difficult to perform.21 In our study, while all patients were successfully intubated, intubating conditions and vocal cord positioning was more optimal in the dexmedetomidine group. 9/48 patients in the lidocaine group reported vocal cord adduction hindering intubation with 44.9% having poor intubating conditions whereas only one patient in dexmedetomidine group had adducted vocal cords at time of intubation. Thus, addition of dexmedetomidine could be a simple and convenient adjunct to nebulised lidocaine for improving intubating conditions.
Better intubating conditions may have resulted in less need of lidocaine supplementation in the dexmedetomidine group. The highest dose of supplemental lidocaine used was 84 mg in this group while it was 150mg in control. Thus, use of dexmedetomidine could have a lidocaine sparing effect and reduce likelihood of local anaesthetic toxicity induced complications. The lidocaine concentrations used in our study were well below the maximal limits advised with the highest dose used being 340mg (5 mg/kg).4 At 6mg/kg of nebulised lidocaine, Parkes et al. always found serum level below a threshold of 5mg/l (highest levels obtained were 0.45mg/l).22
In our study a five-point score was used for assessment of patient comfort, and we found 54.2% (26/48) patients in dexmedetomidine group had no reaction during intubation while 21 patients in lignocaine group had slight grimace and were restless. These findings were similar to those obtained by Kumari et al. who reported superior comfort and satisfaction scores in patient who underwent FOB post dexmedetomidine -lidocaine nebulisation (p<0.001) when compared to lidocaine -fentanyl and plain lidocaine nebulisation.4
Direct laryngoscopy and tracheal intubation are associated with hemodynamic changes due to increased sympathetic activity.23 In susceptible individuals this exaggerated response may precipitate hypertensive crisis, arrhythmias or myocardial ischemia,.24 Dexmedetomidine reduces sympathetic activity by stimulation of postsynaptic 2A receptors in the central nervous system. While Srivastava et al. reported significant blunting of hemodynamic responses to direct laryngoscopy and intubation on use of preoperative nebulised dexmedetomidine, Mishra et al. reported minimal heart rate variation.21, 25 In our study, we found significant attenuation of heart rate and systolic blood pressure at one minute, three-minute, five minute and ten-minute following intubation in patients who received dexmedetomidine nebulisation. Maximum heart rate variability of 4 beats per minute was reported in comparison to 10 beats/minute from baseline value in lidocaine group.
The present study has the following limitations. Due to our existing institutional policy, the study was not randomised and the discretion to include patients in selected groups was left to the individual anaesthetist, which could have contributed to selection bias and affected the results. Another drawback would include the lack of plasma lidocaine level monitoring, a facility not available in our institution.
Conclusion
To summarize, use of dexmedetomidine as adjunct to lidocaine nebulisation facilitates AFOI with better patient comfort, intubating conditions and preserved hemodynamics. Further research into dosing and validation with more randomised controlled studies could provide more insight on the role of this combination in clinical scenarios.